Clinical Practice Article
The efficacy of quadruple therapy in eradicating Helicobacter pylori in children with chronic gastritis: A prospective case series study
Eficacia de la terapia cuádruple en la erradicación de Helicobacter pylori en niños con gastritis crónica: serie de casos prospectiva
Hien Thi Phan1*
https://orcid.org/0000-0002-0235-9135
Ngoan
Thi Dao1
https://orcid.org/0009-0006-8565-9155
Phuong
Minh Nguyen2
https://orcid.org/0000-0003-0948-6871
Phu
Huy Nguyen3
https://orcid.org/0000-0001-6731-618X
Ha
Thu Nguyen4
https://orcid.org/0000-0003-1248-6071
Linh
Thi Thuy Nguyen1
https://orcid.org/0009-0007-1718-2938
1Vinmec
Times City International Hospital. Hanoi City, Vietnam.
2Vietnam
Military Medical Unisversity. Hanoi City, Vietnam.
3Vietnam
National Children's Hospital. Hanoi City, Vietnam.
4National
Institute of Occupational and Environmental Health. Hanoi City, Vietnam.
*Author for correspondence. Email: phanthihienns@gmail.com
Introduction:
Vietnam is a developing country where Helicobacter pylori (H.pylori)
infection and antibiotic resistance rate is high. Then, the first choice
based on triple therapy with standard dose for H. pylori eradication
is low success rate. The quadruple protocol may be more effective for treatment
H. pylori in children with chronic gastritis.
Aim: To evaluate the clinical improvement and the efficacy of quadruple
therapy in eradicating H. pylori in children with chronic non-ulcer gastritis.
Methods: In this prospective case series study, children with gastritis
manifestations underwent upper gastrointestinal endoscopy with urease and histology
tests to determine chronic gastritis and H. pylori infection. All the
patients received a quadruple therapy, including a high dose of esomeprazole
and amoxicillin, combined with metronidazole and bismuth for 2 weeks. 13C-urease
breath test were performed 4 weeks after stopping treatment.
Results: 52 patients aged 5 to 11 years were followed up. The most common
symptoms were abdominal pain (92.3%) and vomiting (42.3%). The others were
less common such as belching (17.3%), epigastric burning (13.5%) and abdominal
distention (13/5%). Asymptomatic children after 4 weeks of treatment stopping
were found in 34 children (58.6%). H. pylori eradication was successful
in 31 of 52 patients (59.6%).
Conclusion:
The quadruple regimen, even with a high dose of esomeprazole and amoxicillin
combined with metronidazole and bismuth, showed a lower eradication rate in
children with chronic gastritis and did not achieve the usual recommended rate
> 90%.
Keywords: children; esomeprazole; gastritis; Helicobacter pylori; quadruple therapy.
Introducción:
Vietnam es un país en desarrollo, con altas tasas de infección por Helicobacter
pylori (H. pylori) y resistencia a los antibióticos. La primera opción
(terapia triple), con dosis estándar para erradicar H. pylori tiene una
baja tasa de éxito. El protocolo cuádruple puede ser más eficaz en niños con
gastritis crónica.
Objetivo: Evalur la mejoría clínica y la eficacia de la terapia cuádruple
en la erradicación de H. pylori en niños con gastritis crónica no ulcerosa.
Métodos: Estudio de serie de casos prospectiva; niños con manifestaciones
de gastritis se sometieron a endoscopia digestiva alta, con pruebas de ureasa
e histología para determinar gastritis crónica e infección por H. pylori.
Los pacientes recibieron terapia cuádruple: Dosis altas de esomeprazol y amoxicilina,
combinadas con metronidazol y bismuto, durante 2 semanas. La prueba del aliento
con 13C-ureasa se realizó 4 semanas después de suspender el tratamiento.
Resultados:
Se realizó seguimiento a 52 pacientes de 5 a 11 años de edad. Síntomas comunes:
Dolor abdominal (92,3 %) y vómitos (42,3 %); menos comunes: Eructos (17,3 %),
ardor epigástrico (13,5 %) y distensión abdominal (13/5 %). Se encontraron asintomáticos
4 semanas después de suspender el tratamiento 34 niños (58,6 %). La erradicación
de H. pylori fue exitosa en 31 de 52 pacientes (59,6 %).
Conclusión:
El esquema cuádruple, incluso con dosis altas de esomeprazol y amoxicilina combinadas
con metronidazol y bismuto, mostró una baja tasa de erradicación en niños con
gastritis crónica y no alcanzó la tasa usualmente recomendada > 90 %.
Palabras clave: esomeprazol; gastritis; Helicobacter pylori;niños; terapia cuádruple.
Received: 16/09/2024
Approved:
30/11/2024
INTRODUCTION
Helicobacter pylori (H. pylori) infection is widely common, more than 50 % in the world,(1)
the rate is higher in developing countries than in developed countries. H. pylori infection is acquired in childhood and remains an important cause of risks later in life such as peptic ulcer disease (PUD) and gastric cancer.
Hence, the recommended goal for H. pylori treatment is an eradication rate of at least 90 %.(2) In fact, there are some causes leading to the low successful rate of H. pylori eradication, including children have presented the low compliance of an H. pylori eradication therapy, side effects while using drugs, the increasing prevalence of antibiotic-resistant strains, and a high rate of re-infection. Therefore, strategy of treatment in children who have non ulcer gastritis associated with H. pylori infection should be based on disadvantages and advantages of the individual child.(3,4,5)
Now, all recommendations showed the compulsory indication for H. pylori eradication in gastric and duodenal ulcer children.(3,4,5) The opinion of this problem in gastritis children without ulcer is variable in different countries. In 2011, the North American and European Society For Pediatric Gastroenterology, Hepatology & Nutrition (NASPGHAN/ESPGHAN) said that pediatricians should discuss with children's parents and explain problems related to eradicating H. pylori. Then, their parents willdecide to approve this treatment or not.(6) The NASPGHAN/ESPGHAN 2016 UPDATE did not officially recommend H. pylori eradication in children with gastritis alone.(4) In Korea, if the cause of abdominal pain or dyspepsia in children with body weights under 35 kg is not found, they need to undergo upper gastrointestinal endoscopy. Then, H. pylori infection is confirmed, they may be treated with aggressive countermeasures which can reduce active and chronic inflammation-mediated adverse effects until they are treated for eradication.(5) In Japan, eradication therapy should be considered in children 5 years of age or older.(3)
Vietnam is a developing country where H. pylori infection and antibiotic resistance rate is high. So, eradication for H. pylori infection may be only useful for some Vietnamese children with non ulcer H. pylori gastritis and high dose PPIs can achieve decrease symptoms. Then, the first choice based on triple therapy with standard dose for H. pylori eradication is high failure rate.(3,4,5) The priority protocol for H. pylori eradication included a high dose of amoxicillin, esomeprazole with metronidazole and bismuth may be had a high rate of H. pylori eradication. There are few studies for this problem in Vietnam. Therefore, this study was conducted in order to evaluate the clinical improvement and the efficacy of quadruple therapy in eradicating H. pylori in children with chronic non-ulcer gastritis.
METHODS
Study design
This study was a prospective case series study with 52 children who were selected according to criteria.
Subjects
Subjects: All consecutive children presenting with digestive symptoms suggestive of gastritis, such as abdominal pain, vomiting, or nausea, who underwent upper gastrointestinal endoscopy at the Vietnam National Children's Hospital in Hanoi, Vietnam, from August 1, 2019, to July 30, 2020, were included in the study.
Inclusion criteria: The study enrolled children aged 5 to 11 years with a diagnosis of H. pylori infection (H. pylori-positive), based on a positive rapid urease test (RUT) and a positive histology result according to the recommendations of NASPGHAN/ESPGHAN 2016,(4) and with inflammation classified according to the Sydney system as active chronic gastritis, i.e., the presence of lymphocytes and neutrophils,(7) who received H. pylori eradication with quadruple therapy and undewent a breath test after treatement, were enrolled in the study.
Non-inclusion criteria: Children with any of the following conditions were excluded from the study: Allergy to any of the drugs used in the study, previous attempts to eradicate H. pylori, receipt of antibiotics, proton pump inhibitors (PPIs), mucosal protective agents, or antacids within 4 weeks of the study, gastro-duodenal ulcer, or evidence of gastritis such as biliary reflux, caustic ingestion, etc.
Losing follow up at 4 weeks since the H. pylori eradication: A total of 57 patients involed in the study. 5 of them were excluded from data anlysis because the discontinued intervention, compliance < 90 % and could not contact. Therefore, there were 52 patients follow up (Fig. 1).
Procedures
The study was carried out in 4 steps to collect data of 52 cases:
1) Step 1, patient eligibility and first monitoring visit (D0): 220 patients' gastroscopy eligibility for enrollment was determined at the hospital based on the verification of all inclusion and non-inclusion criteria for the study, prior to receiving histological results. During endoscopy, performed by an endoscopist using an Olympus GIF-H170 endoscope, biopsies of the gastric body and antrum were taken for RUT and histology tests in each patient. In this step 1, there were 63 cases who did not meet the criteria to proceed to the next step.
2) Step 2, waiting for histological results (D0): 157 patients who showed signs of gastritis during endoscopy and had a positive RUT were prescribed esomeprazole for 2 weeks as routin treatment while awaiting histological results. The researchers recorded information including age, sex, clinical symptoms, endoscopic findings (such as the location and type of lesions), and RUT results. Reseachers intructed parents' patients to complete a questionnaire reporting all clinical symptoms at home. A follow-up appointment was scheduled for day 14. The stomach tissue samples were analyzed using the Giemsa staining technique to detect H. pylori. Additionally, Hematoxylin and eosin staining was used to evaluate inflammatory features of the stomach .
3) Step 3, patients' second monitoring visit for quadruple therapy (D14): Only 122 patients who had the presence of lymphocytes, neutrophils, and H. pylori on gastric mucosal histological examination, i.e., children with active chronic gastritis, continued in the study. Other patients were excluded from the study. The advantages and disadvantages of quadruple therapy which included amoxicillin, metronidazole, bismuth, and esomeprazole (AMBE) were explained to the children and their parents. Once written parental consent was obtained, a complete clinical physical examination was performed for 57 cases. The researchers recorded clinical symptoms for the second time and histological findings such as the grade of chronicity and activity of inflammation. Then they prescribed quadruple therapy for 14 days and distributed a guide explaining the treatment and other monitoring information. Patient's parents filled questionnaire reports about all clinical manifestations, side effects, therapeutic compliance and tolerance of regimen at home. A follow-up appointment was scheduled for the 56th day. A total of 57 cases were involved in the step 3.
4) Step 4, patients' third monitoring visit, 28 days of treatment stopping (D56): 5 out of 57 patients were excluded from data analysis due to discontinued intervention, compliance below 90%, or being unreachable. The patients were examined by researchers. Clinical manifestations and side effects were recorded. Then, the 13C-urease breath test (UBT) for H. pylori was performed for all patients. The 13C UBT was performed using POC machine (Otsuka Electronics, Co., LTD, Japan) with a level of DOB= 2.4/1000 defined as positive. Data from 52 cases were used to analyze the study's results.
In order to avoid biases in the study results, in steps 2, 3 and 4, the other researchers called the patient's parents by hotline on D7, D21, D28, D35, D42 and D49 to to remind them to take notes on clinical manifestations and side effects of the protocol.
AMBE Protocol
All patients received a diet (avoid acidic, spicy foods) and oral AMBE protocols:
- The 0-14th day: Esomeprazole 2 mg/kg/day, maximum 80 mg/day, divided 2 times i.e bid before breakfast and dinner.
- The 15-28th day with quadruple therapy includes:
+ Amoxicillin: 100 mg/kg/day, maximum 3000 mg/day, divided 2 times i.e bid after the meals (tablets 500 mg, sachets 250 mg).
+ Metronidazole: 25 mg/kg/day, maximum 1000 mg/day, divided 2 times i.e bid after the meals (tablets 250 mg).
+ Bismuth subcitrate: 8 mg/kg/day, maximum 480 mg/day, divided 2 times, i.e bid before the meals; and at least 30 minutes from other drugs (tablets 120 mg).
+ Esomeprazole 2 mg/kg/day, maximum 80 mg/day, divided 2 times i.e bid before breakfast and dinner 30 minutes.
Evaluation of treatment results
Baseline demographics and endoscopy and histological features
Clinical efficacy: The clinical efficacy was assessed by the investigators based on the presence or absence of clinical symptoms. All symptoms, such as abdominal pain, nausea and vomiting, hematemesis, belching, epigastric burning, and abdominal distention, were recorded during physical examination or interview at D0 and D56.
Efficacy of H. pylori eradication: All patients were subjected to H. pylori eradication therapy for 14 days. Four weeks after the end of eradication treatment, these patients were confirmed by the 13C-urea breath test (UBT) to determine the status of H. pylori infection. Successful eradication was defined as a negative test result and failure as a positive test.
Adverse events
Vital signs, physical examination was performed. Patient's parents were asked to report any side effects of therapy during of the protocol and were given a possible side effect list, such as nausea and vomiting, diarrhea, constipation and headache. Patients with drug intolerance were excluded who took lower than 90 % of the regimen.
Statistical Analysis
In this study, a case series selected according to criteria. The statistical analysis was performed by using absolute and relative frequency tables. The univariate analysis was conducted by using the McNemar test for categorical variables (Comparing the 2 variables that have been measured in the same cases at different times: D0 and D56). The statistical significance was set at p< 0.05 and all p values quoted. The analysis was conducted using SPSS, version 20.0.
Ethical considerations
The study design and protocol were approved by the Institutional Ethics Committee of the Research Institute of Child Health of Vietnam National Children's Hospital with an authorization number: VNCH - RICH - 2019A68. In addition, a written parental consent was obtained from all mothers and/or fathers in this study.
RESULTS
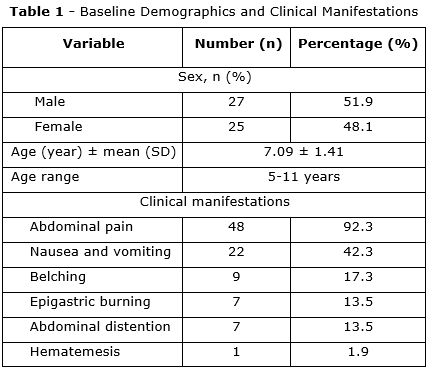
The median age was 7.09 ± 1.41 years (range 5-11 years), with 51.9% being male and 48.1% female (table 1).
Regarding endoscopic lesions, nodules in the antrum were the most common 51/52 patients (98.1%) and then duodenite 23/52 (44.2%). Most patients had moderate and severe chronic inflammation with the rate 34/52 (65,4%) and 17/52 (32,7%), respectively. On activity, the results showed the high rate of moderate and severe activity 35/52 (67.3%) and 6/52 (11.5%) (table 2).
There were 26 patients (50%) without any symptoms after 4 weeks of treatment stopped. The rate of abdominal pain, nausea and vomiting, belching, epigastric burning, abdominal distention was 46.2% (24/52); 3.8% (2/52); 5.8% (3/52); 1.9% (1/52); 1.9% (1/52); respectively (p< 0.05) (table 3).
The study had the 9 cases with side effects, in which 5 cases with headache; 2 vomiting and 2 diarrhea. There were 3 of 9 these patients with a side effect (1 vomiting, 2 diarrhea) could not perform full therapy.
Breath test was performed at 4 weeks of treatment stopping for all patients. H.pylori eradication rate was shown in 31/52 (59.6%) patients (table 3).
DISCUSSION
H. pylori infection plays an important role in children in developing countries with about 70% of infected children until 15 years old. In general, this bacterium is not eliminated without compatible antimicrobial treatment.(8) However, most children are infected without or with mild clinical manifestations such as minor abdominal pain, nausea, vomiting. The study showed that the abdominal pain was predominant with symptoms with the incidence more than > 90 % (table 1). It was equal to the data of Bahremand S et al.(9) and higher other studies of Farahmand F et al. about 60%.(10,11)
Regarding endoscopy findings, almost all patients (98,1%) had antral nodularity on endoscopy that is the typical lesion relative with H. pylori infection (table 2). This result was higher in other studies in Iran,(10,11) Algeria(12) and India(13) because it covered all children infected by H. pylori with or without ulcer on endoscopy. There was one mild haematemesis cases without anemia who also had erosion gastritis. In this research, the subjects were H. pylori infection patients associated with non ulcer gastritis. So, these patients were not in severe condition. Then, many protocols are applied for H. pylori eradication in adults while the treatment is still limited in the younger children with high failure rate.
The table 3 showed the incidence of symptoms clearly decreased at 4 weeks after discontinuation of treatment. H. pylori eradication low rate in the study was 59.6 %, and similarity in somes studies with 14 days of standard triple therapy by lansoprazole+amoxicillin+clarithromycin (ranged from 46.6-68.9%).(14,15) The rate was lower than in others study (75.7-92.5%) used omeprazole or esomeprazole+amoxicillin+clarithromycin(9,10,11,16) or special with sequential treatment-5 days with amoxicillin+omeprazole then 5 days with clarithromycin+omeprazole+tinidazole (97.3%).(17) In the bismuth based on quadruple regimen group, the eradication rates were 84%.(9) In Iran' study demonstrated ciprofloxacin or furazolidone based triple regimen with eradication reported 87.9% or 60.6% in children,(10) but these antibiotics are not recommended in chidlren.
So, the eradication rate is very variable in a large range with triple or quadruple or sequential therapy. Literature showed an adjuvant of probiotics or vitamin E in the standard triple therapy did not increase the eradication efficacy.(14,15,16) Although there are many protocols, the H. pylori eradication rate is still very low in developing countries around 50%. This rate is higher (75%) in developed countries.(14) However, the treatments involve PPI combined with antibiotics and must be successful more than 90% to prevent antibiotic resistance and the risks of rescue protocols.(18) In 2012, Nguyen TVH et al.(19) demonstrated the eradication rate of standard triple therapy in Vietnamese children was 78.2% and 29.3% for clarithromycin sensitive and resistant strains, while 66.7% and 60.3% for metronidazole sensitive and resistant strains. This reaserch noted the high resistance to clarithromycin and metronidazole was 50.9% and 65.3%. In 2023, TC Nguyen CT et al.(20) showed the low eradication rate (56.1%) of triple or quadruple therapy with bismuth in Vietnames children. The H. pylori culture and antibiogram always are available at our hospital but the cost is expensive. So, not all children are eligible for this test before treatment. Therefore, high-dose esomeprazole, amoxicillin, metronidazole, and bismuth were applied in quadruple therapy, but the outcome was very poor.
Current study only recorded 9 out of 55 cases (16.4%) with side effects. These manifestations included 5 cases of headache, 2 of vomiting and 2 of diarrhea. Treatment was discontinued in 3 out of 9 patients: 1 due to vomiting and 2 due to diarrhea. The side effects rate was lower than that in other authors 23.3-38.2% used standard triple or sequential therapy.(16,17,18,19,21)
The study showed, even H. pylori was disappeared, it did not contribute much to clinical improvement. Therefore, H. pylori eradication regimen also is not useful a lot for symptom condition in current in gastritis pre-aldolescent without ulcer. It should be applied to restrict choice after consider carefully advantages, disadvantages and consent of children's parents.
The limitations of the study were that it was mainly a prospective case serises design it difficult to compare the clinical improvement and H. pylori eradication rate. Other limitations are limited knowledge, small sample size, and a short duration for follow-up. Hence, quadruple regimens did not achieve complete clinical improvement and was ineffective for H. pylori eradication. Therefore, a meta-analysis is planned for the future to identify the optimal treatment for gastritis associated with H. pylori in Vietnamese children.
The quadruple regimen, even with a high dose of esomeprazole and amoxicillin combined with metronidazole and bismuth, showed a lower eradication rate in children with chronic gastritis and did not achieve the usual recommended rate > 90%.
BIBLIOGRAPHIC REFERENCES
1. Gao C, Du SY, Fang L, Fan YH, Song AP, Chen H. Eradication treatment of Helicobacter pylori infection based on molecular pathologic antibiotic resistance. Infection drug resistance. 2020;13:69. DOI: 10.2147/IDR.S232169.
2. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143-53. DOI: 10.1136/gut.2009.192757.
3. Kato S, Shimizu T, Toyoda S, Gold BD, Ida S, Ishige T, et al. The updated JSPGHAN guidelines for the management of Helicobacter pylori infection in childhood. J Pediatrics International. 2020;62(12):1315. DOI: 10.1111/ped.14388
4. Jones NL, Koletzko S, Goodman K, Bontems P, Cadranel S, Casswall T, et al. Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (update 2016). Journal of Pediatric Gastroenterology Nutrition 2017;64(6):991-1003. DOI: 10.1097/MPG.0000000000001594.
5. Jun JS, Seo JH, Park JS, Rhee KH, Youn HS. Changes in the treatment strategies for Helicobacter pylori infection in children and adolescents in Korea. Pediatric gastroenterology, hepatology nutrition. 2019;22(5):417. DOI: 10.5223/pghn.2019.22.5.417.
6. Koletzko S, Jones NL, Goodman KJ, Gold B, Rowland M, Cadranel S, et al. Evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children. Journal of Pediatric Gastroenterology and Nutrition 2011;53(2):230-43. DOI: 10.1097/MPG.0b013e3182227e90
7. Sipponen P, Price AB, Hepatology. The Sydney System for classification of gastritis 20 years ago. Journal of Gastroenterology Hepatology. 2011;26:31-4. DOI: 10.1111/j.1440-1746.2010.06536.x.
8. da Silva FAF, de Brito BB, Santos MLC, Marques HS, Sampaio MM, da Silva Júnior RT, et al. Treatment of Helicobacter pylori infection in children: A systematic review. World Journal of Meta Analysis. 2020;8(4):292-308. DOI: 10.13105/wjma.v8.i4.292.
9. Bahremand S, Nematollahi LR, Fourutan H, Tirgari F, Nouripour S, Mir E, et al. Evaluation of triple and quadruple Helicobacter pylori eradication therapies in Iranian children: a randomized clinical trial. European journal of gastroenterology hepatology. 2006;18(5):511-4. DOI: 10.1097/00042737-200605000-00009.
10. Farahmand F, Mohammadi T, Najafi M, Fallahi G, Khodadad A, Motamed F, et al. Comparison of ciprofloxacin-based triple therapy with conventional triple regimen for Helicobacter pylori eradication in children. Acta Medica Iranica. 2016 [access: 21/03/2024];54(6):395-400. Available from: https://pubmed.ncbi.nlm.nih.gov/27306347/
11. Khodadad A, Farahmand F, Najafi M, Shoaran M. Probiotics for the treatment of pediatric helicobacter pylori infection: a randomized double blind clinical trial. Iranian journal of pediatrics. 2013 [access: 17/04/2024]; 23(1):79-84. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3574996/
12. Moubri M, Kalach N, Larras R, Berrah H, Mouffok F, Guechi Z, et al. Adapted first-line treatment of Helicobacter pylori infection in Algerian children. Annals of gastroenterology. 2019;32(1):60. DOI: 10.20524/aog.2018.0317
13. Esmaeili-Dooki MR, Shirdel H, Hajiahmadi M. Eradication of Helicobacter pylori in children by triple therapy regimens of amoxicillin, omeprazole, and clarithromycin or azithromycin. Iranian journal of pediatrics. 2015;25(6):e2360. DOI: 10.5812/ijp.2360
14. Akcam M, Koca T, Salman H, Karahan N. The effects of probiotics on treatment of Helicobacter pylori eradication in children. Saudi medical journal 2015;36(3):286. DOI: 10.15537/smj.2015.3.10124
15. Tümgör G, Baran M, Çakir M, Yüksekkaya HA, Aydogdu S. Comparison of standard and standard plus vitamin E therapy for Helicobacter pylori eradications in children. Turk J Gastroenterol. 2014;25(1):99-103. DOI: 10.5152/tjg.2014.5592
16. Tolone S, Pellino V, Vitaliti G, Tolone C. Evaluation of Helicobacter Pylori eradication in pediatric patients by triple therapy plus lactoferrin and probiotics compared to triple therapy alone. Italian journal of pediatrics. 2012;38(1):1-5. DOI: 10.1186/1824-7288-38-63
17. Francavilla R, Lionetti E, Castellaneta SP, Magistà AM, Boscarelli G, Piscitelli D, et al. Improved efficacy of 10-day sequential treatment for Helicobacter pylori eradication in children: a randomized trial. Gastroenterology. 2005;129(5):1414-9. DOI: 10.1053/j.gastro.2005.09.007
18. Kotilea K, Kalach N, Homan M, Bontems P. Helicobacter pylori infection in pediatric patients: update on diagnosis and eradication strategies. Pediatric Drugs. 2018;20(4):337-51. DOI: 10.1007/s40272-018-0296-y
19. Nguyen TVH, Bengtsson C, Yin L, Nguyen GK, Hoang TTH, Phung DC, et al. Eradication of Helicobacter pylori in children in Vietnam in relation to antibiotic resistance. Helicobacter. 2012;17(4):319-25. DOI: 10.1111/j.1523-5378.2012.00950.x
20. Nguyen CT, Robert A, Pham THA, Vo HK, Le LD, Ma HT et al. Helicobacter pylori Eradication Rate Using Stool Antigen Test in Vietnamese Children: A Prospective Multicenter Study. JPGN Rep. 2023 Nov; 4(4): e374. DOI: 10.1097/PG9.0000000000000374
21. Huang J, Zhou L, Geng L, Yang M, Xu XW, Ding ZL, et al. Randomised controlled trial: sequential vs. standard triple therapy for H elicobacter pylori infection in Chinese children-a multicentre, open-labelled study. Alimentary pharmacology therapeutics. 2013;38(10):1230-5. DOI: 10.1111/apt.12516
Conflict of interest
The authors declare that there is no conflict of interest.
Authors' contributions
Conceptualization: Hien Thi Phan.
Data
curation: Hien Thi Phan, Ngoan Thi Dao.
Formal
analysis: Ngoan Thi Dao, Phuong Minh Nguyen, Phu Huy Nguyen, Ha Thu Nguyen,
Linh Thi Thuy Nguyen.
Research:
Hien Thi Phan, Ngoan Thi Dao, Linh Thi Thuy Nguyen.
Methodology:
Hien Thi Phan, Phu Huy Nguyen.
Supervision:
Hien Thi Phan.
Validation:
Hien Thi Phan, Ngoan Thi Dao, Phu Huy Nguyen.
Writing
- original draft: Hien Thi Phan.
Writing
- review and editing: Hien Thi Phan, Phuong Minh Nguyen.
Data availability
This research data is confidential according to the applicable confidentiality agreements and regulations, therefore, cannot be shared or publicly display. The data are security stored at Research Institute of Child Health of Vietnam National Children's Hospital. Access to these data requires proper authorization. If there are any questions or further information is needed, please contact Hien Thi Phan at phanthihienns@gmail.com