Research Article
Colistin broth disk elution for the detection of Colistin resistance among Carbapenem-resistant Klebsiella pneumoniae
Elución en caldo con disco de colistina para la detección de resistencia a la colistina en Klebsiella pneumoniae resistente a carbapenémicos
Phu Truong Thien1,2
https://orcid.org/0000-0003-0868-2811
Ngoc
Tran Bich1*
https://orcid.org/0000-0002-2255-6398
Hoang
Pham Huy1
https://orcid.org/0009-0008-2090-5268
Van
Tran Thi Hue1
https://orcid.org/0009-0007-0677-2553
Hoang
Phan Thanh1
https://orcid.org/0009-0003-6737-7216
Binh
Pham Thai1
http://orcid.org/0000-0002-2475-8006
Lam
Tran Thi Ngoc1
https://orcid.org/0009-0005-9125-4771
Truc
Nguyen Thi Thanh1
https://orcid.org/0009-0008-6117-4270
Mai
Le Phuong2
https://orcid.org/0009-0001-9625-2522
Dat
Ngo Quoc1
https://orcid.org/0000-0003-1461-0216
1University
of Medicine and Pharmacy at Ho Chi Minh City. Ho Chi Minh City, Vietnam.
2Cho
Ray Hospital. Ho Chi Minh City, Vietnam.
*Author for correspondence. Email: bichngoctran@ump.edu.vn
Introduction:
The emergence of multidrug-resistant pathogens, especially carbapenem-resistant
Klebsiella pneumoniae (CRKP), is a pressing public health issue.
The limited therapeutic choices further compound this. The escalating resistance
to colistin, a crucial last-resort antibiotic, underscores the need for precise
and dependable antimicrobial susceptibility testing (AST) methods.
Objectives:
To evaluate the performance of the Colistin Broth Disk Elution (CBDE)
method in comparison to the reference Broth Microdilution (rBMD) method for
detecting colistin resistance in CRKP isolates.
Methods: A cross-sectional study involving
129 non-duplicate CRKP isolates was conducted at Cho Ray Hospital from October
2023 to December 2023. Colistin susceptibility was assessed using both the
BMD and CBDE methods. The agreement between the two methods was measured in
terms of categorical agreement (CA) and essential agreement (EA) based on
Clinical and Laboratory Standards Institute (CLSI) criteria.
Results: Of the 129 CRKP isolates, 23.26 % were identified
as colistin-resistant by both BMD and CBDE methods. The CA between the two
methods was 100 %, and the EA was 91.47 %, with no major errors (ME) or very
major errors (VME) detected.
Conclusion: The CBDE method has shown a high
level of agreement with the reference BMD method, making it a reliable alternative
for the routine detection of colistin resistance in microbiology laboratories.
The study's findings strongly support using CBDE as a practical and effective
AST method for colistin, thereby contributing to antibiotic stewardship.
Keywords: antimicrobial susceptibility testing; carbapenem resistance; colistin; Klebsiella pneumoniae; Microdilution.
Introducción:
El aumento de patógenos multirresistentes, especialmente Klebsiella
pneumoniae resistente a carbapenémicos (CRKP), representa una preocupación
para la salud pública, agravada por opciones de tratamiento limitadas. La
creciente resistencia a la colistina, un antibiótico de último recurso, resalta
la necesidad de métodos precisos y confiables de pruebas de susceptibilidad
antimicrobiana (AST).
Objetivos: Evaluar la eficacia del método de elución
en caldo con disco de colistina (CBDE) en comparación con el método estándar
de microdilución en caldo (BMD) para detectar resistencia a la colistina en
aislados de CRKP.
Métodos: Estudio transversal con 129 aislados únicos
de CRKP, en el Hospital Cho Ray, desde octubre a diciembre de 2023. La susceptibilidad
a la colistina se evaluó utilizando, tanto los métodos BMD como CBDE. La concordancia
entre métodos se midió en términos de acuerdo categórico (CA) y acuerdo esencial
(EA), basados en los estándares del Instituto de Estándares Clínicos y de
Laboratorio (CLSI).
Resultados: Entre los 129 aislados de CRKP, el 23,26
% se identificaron como resistentes a la colistina por ambos métodos. La CA
entre BMD y CBDE fue del 100 %, con un EA del 91,47 %, y no se observaron errores
mayores (ME) ni muy graves (VME).
Conclusión:
El método CBDE demostró una alta concordancia con el BMD de referencia,
se establece como una alternativa confiable para la detección rutinaria de
resistencia a colistina en laboratorios de microbiología. Estos hallazgos
respaldan el uso de CBDE como un método AST práctico y efectivo para la colistina
y apoya los esfuerzos de gestión de antibióticos.
Palabras clave: colistina; Klebsiella pneumoniae; microdilución en caldo; prueba de susceptibilidad antimicrobiana; resistencia a carbapenémicos.
Received: 30/08/2024
Approved:
25/11/2024
INTRODUCTION
Multidrug resistance is a crucial problem threatening public health worldwide, increasing healthcare costs in diagnostic and therapy. Recently, Enterobacteraleshave received particular attention because of their high resistance to carbapenem and polymyxin B.(1) Among these bacteria, K. pneumoniae is a Gram-negative bacterium commonly found in healthcare settings and is responsible for many infections, such as pneumonia, bloodstream infections, and urinary tract infections.(2) The ability of K. pneumoniae to rapidly acquire and disseminate resistance genes has created multidrug-resistant strains, causing difficulty in treating diseases. Carbapenem-resistant K. pneumoniae (CRKP) has been particularly problematic, often associated with high mortality rates and limited treatment options.(2)
The spreading of CRKP and carbapenem-resistant Enterobacteralesled to the revival of colistin as a last-line treatment, even though its use requires extreme caution.(3) Unfortunately, the use of colistin in both human and veterinary medicine has caused colistin resistance.(4,5) Hence, it becomes necessary that bacteria be identified with colistin resistance in laboratories before considering using it for treatment.(3,4) There is an increasing need for a standardized antimicrobial susceptibility test (AST) for colistin, vital for patient care and surveillance.
The only reference method for colistin AST recognized by the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) is Broth Microdilution (BMD).(6,7) Although BMD is a standard method, it is challenging to do routinely because of its laborious nature. However, CBDE, which has also assessed alternative approaches by CLSI, seems more suitable for routine application in microbiology laboratories due to its relatively simple materials and media.(7,8) In light of this, this study aims to evaluate the performance of the CBDE method compared to the rBMD method for detecting colistin resistance in CRKP isolates.
METHOD
Study design and participants
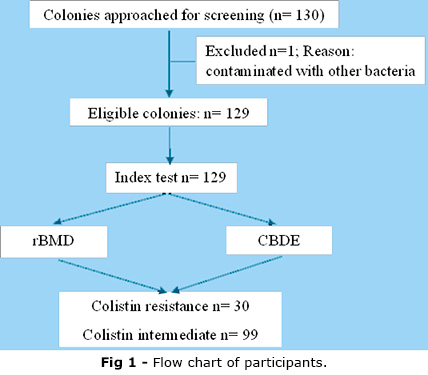
A cross-sectional study was conducted at Cho Ray Hospital from October 2023 to December 2023, with 130 strains of K. pneumoniae collected from routine clinical specimens of blood, urine, sputum, pus, and body fluid. The figure 1 display the flow chart of participants throughout the study duration. These strains resisted at least one carbapenem antibiotic and had colistin-BMD results. Bacterial strains isolated for the second time in the same patient or determined to be from an outbreak, as notified by the infection control department, were excluded from the study. One bacterial strain was excluded due to contamination; there were 129 eligible participant strains.
Data collection
Bacterial strains were identified using the Vitek MS System (BioMérieux, France), and carbapenem-resistant strains were detected with the Vitek-2 Compact System (BioMérieux, France). After assessment, each isolate was preserved in Tryptic Soy Broth (TSB) with 15% glycerol for future use.
Results were recorded and analyzed. The reference BMD was conducted according to the SensitiveTM FRCOL protocol. Cation-adjusted Mueller-Hinton broth procured from Oxoid™ was used for the CBDE method, performed according to the CLSI M100 S34. Colistin sulfate salt was obtained from Oxoid™. Pseudomonas aeruginosa ATCC ® 27853 and Escherichia coli ATCC ® 25922 were used for quality control.
According to CLSI M100 S34, colistin is considered resistant to bacteria strains when the minimum inhibitory concentration (MIC) is = 4 µg/mL and intermediate to bacteria strains when MIC is = 2 µg/mL.
Statistical Analysis
The MIC results of CBDE were compared with the BMD method. The reference BMD test and CBDE agreement were measured in terms of categorical agreement (CA) and essential agreement (EA). CA was determined as the percentage of samples with results in the same category as the reference method, using all tested isolates as the denominator (n= 129). EA was calculated as the percentage of isolates with MIC values within ± 1 log2 dilution or ± 1 twofold dilution of the reference standard. According to CLSI M52 S15, any test with both CA and EA exceeding 90% is considered a reliable alternative to the reference test.(9) Errors were classified as very major errors (VME: false-intermediate) and major errors (ME: false-resistant) by CBDE, with VMEs and MEs of = 3 % deemed unacceptable.
Ethical considerations
The study collected frozen bacterial strains that met the selection criteria without patient intervention. The Ethics Committee of the University of Medicine and Pharmacy in Ho Chi Minh City approved this research under contract number 123/HÐÐÐ-ÐHYD.
RESULTS
Characteristics of the study participants
Table 1 shows that among the 129 CRKP isolates tested in the study, the respiratory tract remains the primary route of infection; 35.7% of CRKP was isolated from phlegm specimens. Most bacterial strains were identified as resistant to ertapenem, imipenem, and meropenem, accounting for 98.4%.
Prevalence of colistin resistance
Table 2 shows the distribution of MIC values in participant strains. The CBDE method, as the BMD method does, cannot determine exactly colistin MIC values at < 1 µg/mL and > 4 µg/mL levels. The first data row represents the MIC breakpoint as per CLSI standards. Of CBDE results, 11 isolates had MIC values exceeding ± 1 two-fold dilution of the reference standard (Fig. 2).
Table 3 shows that this study analyzed 129 CRKP isolates. Of these, 30 out of 129 participant strains were identified as colistin-resistant by the BMD and the CBDE methods, accounting for 23.26 %. No false negative or false positive was detected.
The agreement of CBDE to reference BMD for detecting colistin resistance
Table 4 compares the CBDE results with the MIC values obtained by the BMD method. The CBDE method's CA and EA were within acceptable limits according to CLSI evaluation standards: CA was 100 %, and EA was 91.47 %. No ME or VME was detected in this study. The results suggested that the CBDE method was highly agreeable with the BMD method in detecting colistin resistance.
DISCUSSION
The rise of carbapenem and colistin-resistant pathogens in clinical settings, coupled with the challenges associated with colistin susceptibility testing, is leading to a critical situation in treating severe infections. Implementing the joint CLSI-EUCAST recommended rBMD is challenging in diagnostic clinical microbiology laboratories, highlighting the need to evaluate alternative methods for therapeutic decision-making.(8)
A total of 129 CRKP, isolated from various clinical specimens, were included in this study. Colistin susceptibility was determined using BMD and CBDE methods based on CLSI M52 S15 recommendations.(9) The CBDE method was compared with the reference BMD method based on evaluation criteria recommended by CLSI to assess the concordance between the two methods. The evaluation criteria included CA % and EA % = 90%, and ME % and VME % < 3%.
In this study, 23.26% (30/129) of the CRKP isolates were resistant to colistin, as determined by the reference BMD method. The CBDE method also identified 23.26 % (30/129) of the isolates as colistin-resistant. Based on the collected data, the CBDE method demonstrated a CA % of 100% and an EA % of 91.47%, with no ME or VME recorded. These results indicate that the CBDE method possesses high sensitivity and specificity for determining colistin susceptibility in CRKP and CRE. It is an effective and straightforward method that could be considered a reliable alternative to the BMD test.
Recent studies worldwide have shown results that align well with our findings on the comparability of the BMD and CBDE methods. For instance, a multicenter study was conducted in 2019 to evaluate the efficacy of various testing methods for detecting colistin resistance, as outlined in the CLSI report by Humphries et al.(10) In this study, the CBDE method achieved a CA of 96.8% for 348 Enterobacterales isolates, with VME was 2.5%, while no ME was detected. In comparison, a study by Sujatha et al.(11) in India reported a CA of 99% for the CBDE method, with an ME rate of only 1 % compared to the reference BMD method. Similarly, Kansak N et al.(8) in Turkey observed that the CBDE method achieved a perfect CA of 100%, and, consistent with our findings, no ME or VME was observed. The researchers concluded that the CBDE method demonstrates robust performance compared to the rBMD method, deeming it suitable for routine use in detecting colistin resistance. Furthermore, in a study conducted in India in 2023, Rout et al.(12) reported values of 97.6% for CA, 94.7% for EA, 2.26% for ME, and 1.9% for VME in the CBDE method.
In conclusion, the CBDE method, as implemented in the current study and corroborated by other studies globally, meets the CLSI criteria for comparability with the reference BMD method. The current study provides additional scientific evidence supporting the efficacy of the CBDE method, positioning it as a viable alternative to the BMD test in laboratories of various scales in Vietnam.
There are also some limitations: This research was about the colistin phenotype and showed no mobile cases of colistin resistance. Furthermore, the sample size should come from a multicenter to be more significant in detecting colistin resistance, especially in E. coli.
The CBDE method has shown a high level of agreement with the reference BMD method, making it a reliable alternative for the routine detection of colistin resistance in microbiology laboratories. The study's findings strongly support using CBDE as a practical and effective AST method for colistin, thereby contributing to antibiotic stewardship.
Acknowledgments
We especially thank University of Medicine and Pharmacy at Ho Chi Minh City for funding and Cho Ray Hospital for providing the facilities for this study. Furthermore, we are honored to aid samples from the Microbiology Department, Cho Ray Hospital, in implementing our study.
BIBLIOGRAPHIC REFERENCES
1. Muray CJ, Ikuta KS, Sharara S, Swetschinski L, Aguilar GR, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis [Internet]. The Lancet. 2022; 399(10325):629-55. DOI: 10.1016/S0140-6736(21)02724-0
2. Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasevic AT, et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study [Internet]. The Lancet Infectious Diseases. 2017; 17(2):153-63. DOI: 10.1016/S1473-3099(16)30257-2
3. Bir R, Gautam H, Arif N, Chakravarti P, Verma J, Banerjee S, et al. Analysis of colistin resistance in carbapenem-resistant Enterobacterales and XDR Klebsiella pneumoniae [Internet]. Ther Adv Infect Dis. 2022; 26(9):1-15. DOI: 10.1177/20499361221080650
4. Zhou K, Luo Q, Wang Q, Huang C, Lu H, Rossen JW, el al. Silent transmission of an IS1294b-deactivated mcr-1 gene with inducible colistin resistance [Internet]. International Journal of Antimicrobial Agents. 2018; 51(6):822-8. DOI: 10.1016/j.ijantimicag.2018.01.004
5. Poirel L, Jayol A, Nordmann P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes [Internet]. Clin Microbiol Rev. 2017; 30(2):557-96. DOI: 10.1128/cmr.00064-16
6. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing 34th ed. CLSI supplement M100. Wayne, PA: CLSI; 2024.
7. European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters [Internet]. Version 11.0. Basel: EUCAST; 2021 [access: 28/02/2024]. Available from: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf
8. Kansak N, Arici N, Uzunoner Y, Adaleti R, Aksaray S, Gonullu N. Evaluation of Broth Disk Elution Method to Determine Colistin Resistance in Klebsiella pneumoniae and Escherichia coli Strains [Internet]. Clinical Laboratory. 2023; 69(2): 221008. DOI: 10.7754/clin.lab.2022.221008
9. Clinical and Laboratory Standards Institute (CLSI). Verification of Commercial Microbial Identification and Antimicrobial Susceptibility Testing Systems [Internet]. 15th ed. CLSI supplement M52. Wayne, PA: CLSI; 2015.
10. Humphries RM, Green DA, Schuetz AN, Bergman Y, Lewis S, Yee R, et al. Multicenter evaluation of Colistin Broth Disk Elution and colistin agar test: a report from the Clinical and Laboratory Standards Institute [Internet]. Journal of Clinical Microbiology. 2019; 57(11):10-1128. DOI: 10.1128/jcm.01269-19
11. Sujatha SR, Rajasekhar D, Badveti S, Manthravadi KK. Evaluation of Vitek2 and Colistin broth disk elution test in comparison with Micro broth dilution for Susceptibility testing of Colistin among Carbapenem Resistant Enterobacterales [Internet]. Indian J Public Health Res Dev. 2022; 13(4):290-5. DOI: 10.37506/ijphrd.v14i4.18632
12. Rout B, Dash SK, Kumar Sahu K, Behera B, Praharaj I, Otta S. Evaluation of different methods for in vitro susceptibility testing of colistin in carbapenem-resistant Gram-negative bacilli [Internet]. Access Microbiol. 2023; 5(10): 000595. DOI: 10.1099/acmi.0.000595.v3
Conflict of interest
The authors declare that they have no competing interests.
Financial information
This research was funded by University of Medicine and Pharmacy at Ho Chi Minh City under contract number 69/2024 HÐ-ÐHYD dated 15/04/2024.
Authors' Contributions
Conceptualization: Ngoc Tran Bich, Phu Truong Thien.
Data
curation: Hoang Pham Huy,Ngoc Tran Bich , Phu Truong Thien.
Formal
analysis: Ngoc Tran Bich , Hoang Pham Huy, Lam Tran Thi Ngoc, Truc
Nguyen Thi Thanh, Phu Truong Thien.
Research:
Hoang Pham Huy, Hoang Phan Thanh,Ngoc Tran Bich, Phu Truong Thien.
Methodology:
Hoang Pham Huy, Hoang Phan Thanh,Ngoc Tran Bich, Phu Truong Thien.
Supervision:Phu
Truong Thien , Dat Ngo Quoc, Van Tran Thi Hue.
Validation:Phu
Truong Thien , Ngoc Tran Bich , Binh Pham Thai.
Writing
- original draft: Ngoc Tran Bich, Phu Truong Thien.
Writing
- review and editing:Ngoc Tran Bich, Phu Truong Thien.
Data availability
This research data is confidential according to the applicable confidentiality agreements and regulations and, therefore, cannot be publicly displayed or shared. The data are securely stored at the Integrated Planning Department at Cho Ray Hospital. Access to these data requires proper authorization. If you have any questions or need further information, please contact Ngoc Tran Bich at bichngoctran@ump.edu.vn .