Research Article
Serum epidermal growth factor concentrations in healthy men
Concentraciones séricas de factor de crecimiento epidérmico en hombres sanos
Héctor José Pérez Hernández1*
https://orcid.org/0000-0002-4628-7436
Danay Saavedra Hernández2
https://orcid.org/0000-0002-6614-3819
Tania Crombet Ramos2
https://orcid.org/0000-0002-2550-7292
1Saturnino Lora Provincial Hospital. Santiago de Cuba, Cuba.
2Center of Molecular Immunology. Havana, Cuba.
*Corresponding author. Email: hectorinmunologia@gmail.com
ABSTRACT
Introduction: Despite the documented importance of epidermal growth
factor in health, tiny information is available on its serum
concentrations.
Objective: To describe the behaviour of serum epidermal growth
factor.
Methods: Cross-sectional observational and analytical study in
apparently healthy male subjects selected by simple random sampling from a
group of blood donors. Commercial UMELISA-EGF® kits were used. The
variables used were: sex, age, weight, blood group and serum EGF
concentration. Percentages and arithmetic means were used as summary
measures. Statistical significance was tested using Pearson's chi-squared
test or Welch's t-test with α= 0.05. A univariate strategy was used to
calculate odds ratios.
Results: Serum EGF levels of 507.54±155.69 pg/mL were observed in 32
subjects with a mean age and weight of 34.6±3.82 years and 74.3±3.93 kg,
predominantly blood groups O+ (46.87%) and A+ (28.12%). The probability of
finding EGF levels below 100 pg/mL was higher in subjects <30 years than
in those ≥30 years (OR=4.2 CI_95%: 0.8783-3.9599; p=0.0007). The
probability of finding EGF levels below 870 pg/mL is higher in A+ than in
O+ subjects (OR=4 CI_95%: 0.6840-4.8739; p=0.0171).
Conclusions: There is evidence of a putative association between
serum EGF levels and A+ and O+ blood groups, with an apparent inverse
association with body weight. The variability of serum EGF concentrations
decreases with increasing age.
Keywords: epidermal growth factor; healthy volunteers; men.
RESUMEN
Introducción: Del factor de crecimiento epidérmico, a pesar de su
relevancia documentada en salud, se dispone de escasa información, respecto
de sus concentraciones séricas.
Objetivo: Describir el comportamiento del factor de crecimiento
epidérmico sérico.
Método: Estudio observacional transversal y analítico, en sujetos
aparentemente sanos del sexo masculino, seleccionados por muestreo
aleatorio simple de un grupo de donantes de sangre. Se emplearon los kits
comerciales de UMELISA-EGF. Las variables fueron: sexo, edad, peso, grupo
sanguíneo y concentración sérica de EGF. Las medidas resumen empleadas
fueron el porcentaje y la media aritmética. La significación estadística se
exploró con la prueba de ji cuadrado de Pearson o la prueba t de
Welch con α= 0,05. Para determinar el odds ratio se utilizó una
estrategia univariada.
Resultados: Se observaron niveles séricos de EGF de 507,54 ± 155,69
pg/mL en 32 sujetos con una edad y peso medios de 34,6 ± 3,82 años y 74,3 ±
3,93 kg, predominantemente de los grupos sanguíneos O+ (46,87 %) y A+
(28,12 %). La probabilidad de encontrar niveles de EGF inferiores a 100
pg/mL fue mayor en sujetos < 30 años que en aquellos ≥ 30 años (OR= 4,2
IC95 %: 0,8783-3,9599; p= 0,0007). La probabilidad de encontrar niveles de
EGF inferiores a 870 pg/mL es mayor en sujetos A+ que en O+ (OR= 4 IC95 %:
0,6840-4,8739; p= 0,0171).
Conclusiones: Existen indicios de una presumible relación de los
valores séricos de EGF en relación con grupos sanguíneos A+ y O+, con una
aparente relación inversa con el peso corporal. La variabilidad de las
concentraciones sérica de EGF se reduce a medida que se avanza en edad.
Palabras clave: factor de crecimiento epidérmico; hombres; voluntarios sanos.
Recibido: 21/09/2024
Aprobado: 06/06/2025
INTRODUCTION
Signaling through the epidermal growth factor receptor (EGFR) is involved in the regulation of multiple biological processes. It includes proliferation, metabolism, differentiation and cell survival,(1) where intracellular downstream signaling through the mitogenesis activating protein kinase (MAPK) and signal transducer, and activator of transcription 3 (STAT3) pathway are crucial;(2,3,4) they are at the crossroads between the processes of growth, inflammation and damage repair.
Although several ligands can bind to this receptor, such as transforming growth factor (TGF)-α, amphiregulin (AREG) and epiregulin (EREG), its canonical ligand is epidermal growth factor (EGF). Discovered and characterized by Stanley Cohen(5) between 1962 and 1975, this molecule was extensively studied in the following years, with approaches to its role in growth physiology, with special emphasis on its role as a growth mediator.(6,7,8,9)
EGF is the term by which a 53 amino acid (a.a.) polypeptide, with a molecular weight of 6045 Daltons (Da), proteolytically derived from the prepro-EGF transmembrane protein of about 1207 a.a. in humans, encoded on the long arm of chromosome four (4q25) is known.(10) The human EGF gene is approximately 110 kilobase pairs long, and has 24 exons, where several EGF precursor domains are encoded by individual exons, and in addition, 15 of the 24 exons encode protein segments that are homologous to sequences in other proteins, such as transferrin and LDL receptors.(11)
A special motif, critical for inducing homodimerization of EGFR (HER1-HER1), although it also induces heterodimerization of EGFR (HER1-HER2), is organized as in the cysteine pattern CX7CX4-5CX10CXCXCX8C (X can be any amino acid) in the cytoplasmic domain, approximately 25 residues from the transmembrane domain. Of all the proteins containing this motif only EGF has nine motifs,(12,13,14) of which only the one adjacent to the cell membrane has a binding function to the EGFR domain.(15,16)
The protein has been associated to multiple diseases, from entities of degenerative nature to cancer,(17,18,19) including a special participation related to the life cycle and replicability of viruses, however, most of the approaches are inferred based on the fact that its receptor is preferentially studied.(20,21) However, experiences of therapeutic use of the molecule stand out as positive. An example of this is the diabetic foot ulcers that cause a high rate of amputations in diabetics, where the use of Heberprot-P® (recombinant EGF) revolutionized its treatment.(22,23,24)
Therefore, the availability of data on EGF concentrations in biological fluids is not only important for the definition of its value as a predictor, but could also contribute to a better understanding of its role, beyond its classic functions.
In view of the above, it is proposed to describe the behavior of serum concentrations of EGF in apparently healthy male subjects.
METHODS
Analytical cross-sectional observational study, in apparently healthy male subjects, selected by simple random sampling from a group of blood donors according to their willingness to participate in the study and the medical confirmation of their optimal health status, recruited between June and December 2022, at the Renato Guitar Provincial Blood Bank, in Santiago de Cuba.
No additional selection criteria were established, assuming the quality management and donor selection procedures, as models of healthy subjects, in accordance with Ministry of Public Health of Cuba (Minsap) Resolution No. 101/2008: Requirements for the Selection of Blood Donors.
As an analytical method for the quantification of serum EGF, the UMELISA-EGF® commercial kits, of SUMA technology, produced and marketed by the Cuban Immunoassay Center, were used in the certified laboratories of the "Juan Bruno Zayas Hospital" in Santiago de Cuba. The results of the enzymatic assay were expressed in pg/mL. Age, sex, blood group and body weight were recorded as main control variables.
The clinical data were retrieved from the medical records, and organized in a digital database using the services of the Microsoft Office Excel calculation platform.
For the statistical description, the summary measures used were the arithmetic mean and median as parameters of central tendency; the standard deviation and variance as measures of dispersion, as well as other symmetry and linear correlation coefficients: coefficient of skewness, kurtosis, Pearson correlation coefficient and first-order partial correlation coefficient. For the summary of the qualitative aspects, the results were expressed as percentages.
For normality tests, Q-Q probability distribution plots and the Jarque-Bera (JB) test were used. In the statistical analysis, graphical and computational means were used to determine the normality of the data distribution, as well as to explore statistical significance, Fischer's test and Welch's t-testwere used assuming α= 0.05, and Hedges' g was used to estimate the effect size. A univariate strategy was used to calculate odds ratios (OR). Cohen´s d effect size estimator was used.
Informed consent was obtained from the subjects for sample collection and analysis, and the research was carried out under the spirit and letter of the Declaration of Helsinki and the Document of Good Clinical Practices for the Americas.
RESULTS
The sample consisted of a total of 32 male subjects with a health history. The average age 34.6 ± 3.82 years, for an age range of 20-59 years (JB=2.6455; p= 0.2663), with 50% of the subjects under 30 years of age. Mean weight was 74.3 ± 3.93 kg for a weight range of 60-104 kg (JB= 3.4901; p= 0.1746). The predominant blood groups were O+ and A+, with a frequency of 46.87% and 28.12% respectively.
The average serum EGF levels were 507.54 ± 155.69 pg/mL in a range of 39-1725.73 pg/mL (JB= 4.6063; p= 0.0999). The exploration of correlations did not show a notable correlation between EGF values with respect to age (r= -0.05; p= 0.3937) and with respect to weight in a negative sense (r= -0.3261; p= 0.0656).
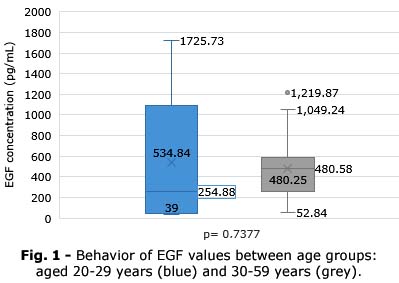
Behavior of EGF values between age groups it is illustrated in figure 1.
An analysis in the group of subjects aged 20-29 years (n=16), with a mean age 25.5± 1.49 years and mean weight 75.5± 6.15 kg, showed a mean EGF 534.8 ± 275.6 pg/mL with a median of 254.8 pg/mL. Exploration of correlates reflected similar trends to those already observed (rEGF-weight= -0.4226; p=0.1030).
On the other hand, in the group of subjects aged 30-59 years (n= 16) mean age 43.7 ± 4.00 years and mean weight 73.1± 5.04 kilograms (kg), mean EGF 480.24 ± 154.4 pg/mL with a median of 480.5 pg/mL was observed. In the correlates scan, there was a loss of the notable relationship between EGF and weight previously observed (rEGF-weight= -0.1615; p=0.2919), with all other relationships unnoticed. The probability of finding EGF values below 100 pg/mL is higher in subjects younger than 30 years than in those older than 30 years (OR=4.2; 95% CI: 0.8783-3.9599; p= 0.0007).
Behavior of EGF values between weights it is illustrated in figure 2.
In the group of men weighing less than 75 kg, the mean weight was 66.8 ± 2.78 kg, with a mean age of 34.05 ± 4.73 years, with EGF values 556.5 ± 237.7 pg/mL with a median of 388.7 pg/mL. In the correlates scan, there was a loss of the remarkable relationship between EGF and weight previously observed (rEGF-weight= -0.2554; p= 0.1886).
In the group of men with weight greater than or equal to 75 kg, the mean weight was 84± 4.78 kg, with a mean age of 35.4± 6.48 years, with EGF values 444.5±186.9 p/mL with a median of 432.6 pg/mL. In the correlates scan, there was a recovery and increase in the remarkable relationship between EGF and weight previously observed (rEGF-weight= -0.5449; p=0.0773).
Behavior of EGF values between blood groups (A+ vs O+). it is illustrated in figure 3.
In the group of men with blood group A+, the mean weight was 76.3± 8.11 kg, with a mean age of 35.8± 6.42 years, with EGF values 417.6±250.9 pg/mL with a median of 391.7 pg/mL. In the correlates exploration, the remarkable relationship between EGF and weight was observed (rEGF-weight= -0.3161; p= 0.2448).
In the group of men with blood group O+, the mean weight was 73.4 ± 6.39 kg, with a mean age of 34.1 ± 5.56 years, with EGF values 593.5 ± 266.3 pg/mL with a median of 479.7 pg/mL. In the correlates scan, there was no notable correlation between EGF and weight (rEGF-weight= -0.2814; p= 0.1931). The probability of finding EGF values below 870 pg/mL is higher in A+ than in O+ subjects (OR=4; 95% CI: 0.6840-4.8739; p= 0.0171).
DISCUSSION
Despite the relevance of EGFR to current medical practice, systematic studies that delve into the biology of its canonical ligand remain scarce in the literature. Few studies address the behavior of serum concentrations in healthy subjects. This study provides new data concerning the behavior of EGF in apparently healthy male subjects, exploring possible correlations with other clinical parameters.
In general, the role of this growth factor in activating deoxyribonucleic acid synthesis, cell proliferation, and stimulating mitogenesis in epidermal tissue, as well as participating in angiogenesis of epidermal tissue, is well established. Although its overexpression has been reported to directly impact on accelerated cell differentiation and proliferation, inhibition of apoptosis and enhancement of the invasiveness of cancer cells in many types of epithelial tumors.(25)
Although transcriptomic analyses point to a very intense activity at the renal level (30 transcripts per million (pTPM)), compared to the rest of the tissues and cells of our body a subgroup of neutrophils, widely spread, expresses a transcriptomic profile markedly superior to the rest of the cells of the immune response.(26) Recent studies in the context of COVID-19 have reported similar trends, extending the observations to other classical pro-inflammatory cytokines such as IL-1β and MIP-1a (CCL3), as well as cytokines associated with Th2 (IL-4, IL-5 and IL-13) and Th17 (IL-17A, IL-17F, IL-17E/IL-25 and IL-22) polarization.(27) All of which points to indicate an effect of EGF beyond the direct interaction with its classical receptor.
Levels established as standard or normal for serum EGF concentrations are not clearly defined. Among the few examples of studies that explore serum EGF concentrations, was found a great diversity of results. It is therefore necessary to deepen in this type of studies, given the medical importance in inflammatory contexts, with particular interest in pneumopathies of infectious origin. It is imperative to develop this line of research, as a comprehensive characterisation of serum values could significantly contribute to subsequent correlations. These correlations could serve as a therapeutic objective, which is particularly salient in the context of pneumopathies of inflammatory nature and the ongoing global pandemic of the Coronavirus.
A study developed in Belgium is one of the few published, where in a small sample of children and adults, it was observed that levels higher than 1000 pg/ml in children (n=78) and up to 400 pg/mL in adults (n= 50) were not related to pathological processes.(28) Przepiera-Bedzak H et al.(29) reported for the healthy controls of their study (n= 20) EGF values of 93.0 pg/mL (45.0-192.5). On the other hand, Serilmez M et al.(30) reported for the same number of controls a mean EGF of 465.3 ± 172.2 pg/mL (304-706.6) with a median of 479.2 pg/mL. Przepiera-Bedzak H et al.(29) used ELISA kit, Human EGF Immunoassay Quantikine®, which was used as a control for the validation of the kit developed by the Cuban Immunoassay Center; as well as Meybosch S et al.(28) used the EGF human Elisa kit (Invitrogen, California, USA), both comparable technologies.
One of the most complete studies published on serum EGF concentrations is that of Pérez-González I et al.;(31) in this study for the particular case of men between 18 and 45 years of age, with a mean of 36.67 ± 1.83 years (n= 21), a mean EGF value of 376.40 ± 51.34 pg/mL, with a median of 323 pg/mL is reported, as well as for men between 46 and 50 years of age, with a mean of 53.22 ± 1.02 years (n= 18), a mean EGF value of 402.70 ± 54.03 pg/mL is reported with a median of 387.2 pg/mL.(31)
Comparative analysis of our data, in general, shows no significant differences with respect to the results obtained by Serilmez M et al.(30) (d= 0.0853), although a slight difference in effect is observed with that reported by Perez-González Iet al.(31) (d= 0.3149). Our results reflected a greater variability of EGF in subjects younger than 30 years, which is a relatively expected tendency according to the known biology of the molecule and coincides with what is reported in the literature.
It was striking the establishment of remarkable correlations between body weight and EGF values, with a negative sense of relationship, more marked in those over 84 kg body weight, on average, with respect to subjects under 30 years of age, and only observed in these two particular cases. A possible explanation may lie in the convergence between obesity and EGFR expression in the inflammatory context, with a possible effect of apparent reduction in periphery observable by a relative increase in the consumption of the molecule.
Likewise, we observed a greater dispersion of data associated with blood group O+ with respect to A+; the absence of reports exploring possible differences in EGF concentrations associated with blood group limits the comparison of these results.
The large differences in the results, which are based on the same technological platform with similar sensitivity thresholds, could be due, in the authors' opinion, to differences in the processing of the sample, which could be responsible for these observable differences among the studies.
In this study, the reported trends were replicated respect the variability of serum EGF concentrations with advancing age. Systematic studies, with a larger number of subjects and with a robust statistical design, are essential to establish patterns of normality of EGF in the context of apparent health status, as well as to elucidate possible correlations with both analytical parameters and other clinical variables.
There is evidence of a putative association between serum EGF levels and A+ and O+ blood groups, with an apparent inverse association with body weight. The variability of serum EGF concentrations decreases with increasing age.
BIBLIOGRAPHIC REFERENCES
1. Noh SS, Shin HJ. Role of Virus-Induced EGFR Trafficking in Proviral Functions [Internet]. Biomolecules. 2023; 13(12): 1766. DOI: 10.3390/biom13121766
2. Levantini E, Maroni G, Del Re M, Tenen DG. EGFR signaling pathway as therapeutic target in human cancers [Internet]. Seminars in cancer biology. 2022; 85: 253-275. DOI: 10.1016/j.semcancer.2022.04.002
3. Sabbah DA, Hajjo R, Sweidan K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors [Internet]. Current Topics in Medicinal Chemistry. 2020; 20(10): 815-34. DOI: 10.2174/1568026620666200303123102
4. Feng J, Hu Z, Xia X, Liu X, Lian Z, Wang H, et al. Feedback activation of EGFR/wild-type RAS signaling axis limits KRASG12D inhibitor efficacy in KRASG12D-mutated colorectal cancer [Internet]. Oncogene. 2023; 42(20): 1620-33. DOI: 10.1038/s41388-023-02676-9
5. Tarvestad-Laise KE, Ceresa BP. Modulating Growth Factor Receptor Signaling to Promote Corneal Epithelial Homeostasis [Internet]. Cells. 2023; 12(23): 2730. DOI: 10.3390/cells12232730
6. Patnaik SK, Chandrasekar MJN, Nagarjuna P, Ramamurthi D, Swaroop AK. Targeting of ErbB1, ErbB2, and their Dual Targeting Using Small Molecules and Natural Peptides: Blocking EGFR Cell Signaling Pathways in Cancer: A Mini-Review [Internet]. Mini reviews in medicinal chemistry. 2022; 22(22): 2831-46. DOI: 10.2174/1389557522666220512152448
7. Murphrey MB, Quaim L, Rahimi N, Varacallo M. Biochemistry, Epidermal Growth Factor Receptor. Treasure Island: StatPearls Publishing; 2023. [acceso: 31/10/2024]. Disponible en: https://www.ncbi.nlm.nih.gov/books/NBK482459/
8. Torres-Castro P, Grases-Pintó B, Abril-Gil M., Castell M, Rodríguez-Lagunas M, Pérez-Cano J, et al. Modulation of the Systemic Immune Response in Suckling Rats by Breast Milk TGF-β2, EGF and FGF21 Supplementation [Internet]. Nutrients. 2020; 12(6): 1888. DOI: 10.3390/nu12061888
9. Turner JM, George P, Lansing M, Slim G, Wizzard PR, Nation P, Brubaker PL, Wales PW. In the Short-term, Milk Fat Globule Epidermal Growth Factor-8 Causes Site-specific Intestinal Growth in Resected Piglets [Internet]. Journal of pediatric gastroenterology and nutrition. 2020; 71(4):543-9. DOI:10.1097/MPG.0000000000002818
10. Carpenter G, Cohen S. Epidermal growth factor [Internet]. Annu Rev Biochem. 1979 [acceso: 31/10/2023];48(1):193-216. Disponible en: https://pubmed.ncbi.nlm.nih.gov/382984/
11. da Rocha JF, Bastos L, Domingues SC, Bento AR, Konietzko U, da Cruz E Silva O. AB, Vieira SI. APP Binds to the EGFR Ligands HB-EGF and EGF, Acting Synergistically with EGF to Promote ERK Signaling and Neuritogenesis [Internet]. Molecular neurobiology. 2021; 58(2):668-88. DOI:10.1007/s12035-020-02139-2
12. Leblanc JA, Sugiyama MG, Antonescu CN, Brown AI. Quantitative modeling of EGF receptor ligand discrimination via internalization proofreading [Internet]. Physical biology. 2023; 20(5): 056008. DOI:10.1088/1478-3975/aceecd
13. Liu S, Wang Y, Han Y, Xia W, Zhang L, Xu S, et al. EREG-driven oncogenesis of Head and Neck Squamous Cell Carcinoma exhibits higher sensitivity to Erlotinib therapy [Internet]. Theranostics. 2020 [acceso: 31/10/2023];10(23):10589-605. Disponible en: https://pubmed.ncbi.nlm.nih.gov/32929368/
14. Pascarelli, S, Merzhakupova D, Uechi GI, Laurino P. Binding of single-mutant epidermal growth factor (EGF) ligands alters the stability of the EGF receptor dimer and promotes growth signaling [Internet]. The Journal of biological chemistry. 2021; 297(1): 100872. DOI:10.1016/j.jbc.2021.100872
15. Sperrhacke M, Leitzke S, Ahrens B, Reiss K. Breakdown of Phospholipid Asymmetry Triggers ADAM17-Mediated Rescue Events in Cells Undergoing Apoptosis [Internet]. Membranes. 2023; 13(8):720. DOI:10.3390/membranes13080720
16. Cheng W-L, Feng P-H, Lee K-Y, Chen K-Y, Sun W-L, Van Hiep N, et al. The role of EREG/EGFR pathway in tumor progression. [Internet]. Int J Mol Sci. 2021 [acceso: 31/10/2023];22(23):12828. Disponible en: https://pubmed.ncbi.nlm.nih.gov/34884633/
17. Greten FR, Grivennikov SI. Inflammation and cancer: Triggers, mechanisms, and consequences [Internet]. Immunity. 2019 [31/10/2023]; 51(1):27-41. Disponible en: https://pubmed.ncbi.nlm.nih.gov/31315034/
18. Cuesta AM, Palao N, Bragado P, Gutierrez-Uzquiza A, Herrera B, Sánchez A, Porras A. New and Old Key Players in Liver Cancer [Internet]. International journal of molecular sciences. 2023; 24(24):17152. DOI:10.3390/ijms242417152
19. Kalinowski A, Galen BT, Ueki IF, Sun Y, Mulenos A, Osafo-Addo A, et al. Respiratory syncytial virus activates epidermal growth factor receptor to suppress interferon regulatory factor 1-dependent interferon-lambda and antiviral defense in airway epithelium [Internet]. Mucosal Immunol. 2018 [31/10/2023];11(3):958-67. Disponible en: https://pubmed.ncbi.nlm.nih.gov/29411775/
20. Lu J, Xing H, Wang C, Tang M, Wu C, Ye F, et al. Mpox (formerly monkeypox): pathogenesis, prevention, and treatment [Internet]. Signal transduction and targeted therapy. 2023; 8(1):458. DOI:10.1038/s41392-023-01675-2
21. Murphrey MB, Quaim L, Varacallo M. Biochemistry, epidermal growth factor receptor [Internet]. Treasure Island: StatPearls Publishing; 2023. [31/10/2023]. Disponible en: https://pubmed.ncbi.nlm.nih.gov/29494066/
22. Akkus G, Sert M. Diabetic foot ulcers: A devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities [Internet]. World journal of diabetes. 2022;13(12):1106-1121. DOI:10.4239/wjd.v13.i12.1106
23. Özker E. Intralesional epidermal growth factor therapy in recalcitrant diabetic foot ulcers [Internet]. J Wound Care. 2023 [31/10/2023]; 32(Sup4):S14-21. Disponible en: https://pubmed.ncbi.nlm.nih.gov/37029977/
24. Ilkeli E, Fb GD, Duzgun AC, Arabaci H, Uysal A, Kanko M. Intralesional epidermal growth factor for diabetic foot ulcers [Internet]. J Coll Physicians Surg Pak. 2022 [31/10/2023]; 32(3):278-82. Disponible en: https://pubmed.ncbi.nlm.nih.gov/35148575/
25. Holbro T. The ErbB receptors and their role in cancer progression [Internet]. Exp Cell Res. 2003 [31/10/2023];284(1):99-110. Disponible en: https://pubmed.ncbi.nlm.nih.gov/12648469/
26. Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, et al. Towards a knowledge-based Human Protein Atlas [Internet]. Nature Biotecnology. 2010;28:1248-50. DOI: 10.1038/nbt1210-1248
27. Kalinina O, Golovkin A, Zaikova E, Aquino A, Bezrukikh V, Melnik O, et al. Cytokine storm signature in patients with moderate and severe COVID-19 [Internet]. Int J Mol Sci. 2022 [31/10/2023];23(16):8879. Disponible en: https://pubmed.ncbi.nlm.nih.gov/36012146/
28. Meybosch S, De Monie A, Anné C, Bruyndonckx L, Jürgens A, De Winter BY, et al. Epidermal growth factor and its influencing variables in healthy children and adults [Internet]. PLoS One. 2019 [31/10/2023];14(1):e0211212. Disponible en: https://pubmed.ncbi.nlm.nih.gov/30677083/
29. Przepiera-Będzak H, Fischer K, Brzosko M. Serum levels of angiogenic cytokines in psoriatic arthritis and SAPHO syndrome [Internet]. Pol Arch Med Wewn. 2013 [31/10/2023];123(6):297-302. Disponible en: https://pubmed.ncbi.nlm.nih.gov/23711571/
30. Serilmez M, Özgür E, Karaman S, Gezer U, Duranyıldız D. Detection of serum protein and circulating mRNA of cMET, HGF EGF and EGFR levels in lung cancer patients to guide individualized therapy [Internet]. Cancer Biomark. 2019 [31/10/2023];25(2):177-84. Disponible en: https://pubmed.ncbi.nlm.nih.gov/31104010/
31. Perez-González I, Haslen Hassiul CL, Adriana CP, Monzon Kalet L. Measurement of serum EGF levels, a methodological approach: Learning what means "low-/high-concentration of EGF in serum". Some clinical implications [Internet].J Mol Biomark Diagn. 2017 [31/10/2023];08(03):1-8. Disponible en: https://www.europeanbionetwork.com/abstract/jm-bd/measurement-of-serum-egf-levels-a-methodol-ogical-approach-learning-what-means-8220lowhighconcentration-of-egf-in-serum82-37145.html
Conflict of interest
The authors declare that they have no conflicts of interest with the research conducted.
Authorship contribution
Conceptualization: Héctor José Pérez Hernández.
Data curation: Héctor José Pérez Hernández.
Formal analysis:
Héctor José Pérez Hernández, Danay Saavedra Hernández, Tania Crombet
Ramos.
Research: Héctor José Pérez Hernández, Tania Crombet Ramos.
Methodology:
Héctor José Pérez Hernández, Danay Saavedra Hernández, Tania Crombet
Ramos.
Project Management:
Héctor José Pérez Hernández, Tania Crombet Ramos.
Supervision: Danay Saavedra Hernández, Tania Crombet Ramos.
Validation: Danay Saavedra Hernández, Tania Crombet Ramos.
Visualization: Héctor José Pérez Hernández, Tania Crombet Ramos.
Editor - original draft: Héctor José Pérez Hernández.
Writing - revision and editing:
Héctor José Pérez Hernández, Tania Crombet Ramos.
Data availability statement
The study data are confidential, to access them, authorization is required from the General Management of the "Saturnino Lora" Provincial Hospital.