Research Article
Quality of the fibrinogen index in freeze-dried plasma for external blood coagulation testing programs
Calidad del índice de fibrinógeno en plasma liofilizado para programas de pruebas externas de coagulación sanguínea
Thi Hong Nguyen1,2
https://orcid.org/0000-0001-9565-7205
Thanh
Tung Tran3
https://orcid.org/0009-0005-6770-2298
Phuc
Thi Diem Huynh1
https://orcid.org/0000-0002-8173-4115
Quang
Huy Vu4*
https://orcid.org/0000-0003-3509-1814
1University
of Medicine and Pharmacy at Ho Chi Minh City. Ho Chi Minh City, Vietnam.
2University
of Medicine and Pharmacy. Department of Nursing and Medical Technology. Can
Tho City, Vietnam.
3Cho
Ray Hospital. Department of Hematology. Ho Chi Minh City, Vietnam.
4Melatec
General Hospital. Department of Laboratory Medicine. Ho Chi Minh City, Vietnam.
*Corresponding author. Email: drvuquanghuy@gmail.com
Background:
Lyophilized plasma is widely used in external quality assessment for fibrinogen
testing, where sample homogeneity and stability are critical. This study evaluated
these factors, addressing challenges in sample preparation to enhance the quality
of medical services.
Objective: To assess the homogeneity and stability of fibrinogen in lyophilized
plasma according to ISO 13528:2022 and ISO Guide 35:2010 standards.
Methods:
The study utilized experimental freeze-dried control samples containing fibrinogen.
Random sampling was performed using Microsoft Excel 16, and one-way ANOVA was
applied to assess sample homogeneity. Stability at different time points and
temperatures was evaluated using t-tests. Key variables included: Mean (central
tendency), Standard Deviation (data dispersion), Homogeneity (consistency within
a batch, assessed via P-values), and Stability (maintenance of values over time
under varied storage conditions).
Results:
The fibrinogen values of the standard samples demonstrated good homogeneity
(p= 0.890> 0.05). Freeze-dried samples stored at 2-8°C remained stable over
5 months, with t-test p-values of 0.114, 0.242, and 0.391, all > 0.05. Samples
stored at -20°C were stable over 3 months (p= 0.520, 0.761, and 0.353). Additionally,
short-term stability assessments on days 1, 3, 5, and 7 yielded p-values of
0.093, 0.742, 0.057, and 0.270, respectively, indicating no significant changes
and confirming overall stability.
Conclusion:
Lyophilized plasma containing the fibrinogen index is both homogeneous and stable
when stored at 2-8°C and -20°C, as well as during transportation. These significant
findings pave the way for further research and potential advancements in coagulation
testing.
Keywords: coagulation; quality assurance; fibrinogen; lyophilized; plasma.
Antecedentes:
El plasma liofilizado se utiliza en la evaluación externa de la calidad
de las pruebas de fibrinógeno, pues homogeneidad y estabilidad de la muestra
son fundamentales.
Objetivo:
Evaluar la homogeneidad y estabilidad del fibrinógeno en plasma liofilizado,
según las normas ISO 13528:2022 e ISO Guía 35:2010.
Métodos:
Se utilizaron muestras de control experimentales liofilizadas con fibrinógeno.
Se realizó muestreo aleatorio y se aplicó ANOVA de una vía para evaluar homogeneidad.
La estabilidad en diferentes momentos y temperaturas se evaluó mediante pruebas
t. Las variables fueron: Media, desviación estándar, homogeneidad (consistencia
dentro de un lote, evaluada mediante valores p) y estabilidad (mantenimiento
de los valores a lo largo del tiempo en diversas condiciones de almacenamiento).
Resultados:
Los valores de fibrinógeno de las muestras estándar mostraron buena homogeneidad
(p= 0,890> 0,05). Las muestras liofilizadas almacenadas a 2-8 °C se mantuvieron
estables durante 5 meses (prueba t con p= 0,114, 0,242 y 0,391; todos > 0,05).
Las muestras almacenadas a -20 °C se mantuvieron estables durante 3 meses (p=
0,520, 0,761 y 0,353). Las evaluaciones de estabilidad a corto plazo, realizadas
los días 1, 3, 5 y 7 arrojaron valores p= 0,093, 0,742, 0,057 y 0,270, respectivamente,
lo que no indicó cambios significativos y confirmó la estabilidad general.
Conclusión:
El plasma liofilizado con índice de fibrinógeno es homogéneo y estable, tanto
al almacenarse a 2-8 °C, como a -20 °C, y durante el transporte. Estos hallazgos
abren el camino a futuras investigaciones y posibles avances en las pruebas
de coagulación.
Palabras clave: coagulación; evaluación externa de calidad; fibrinógeno; liofilizado; plasma.
Received: 17/02/2025
Approved:
06/06/2025
INTRODUCTION
Anemia, caused by factors like alcohol use, sickle cell disease, thalassemia, chronic disease, iron deficiency, and lead poisoning, occurs when the body lacks essential nutrients to produce red blood cells. This impairs oxygen transport, hindering recovery and increasing infection risk.(1,2) Zinc deficiency is known to have a significant impact on the immune system and various physiological functions.(3) A study by La Qui Phu et al. reported a significantly higher rate of anemia in children with pneumonia and zinc deficiency (37%) compared to those without zinc deficiency (19.3%) (p< 0.05).(4) This predisposes patients with pneumonia to complications, weakens the immune system, and increases the risk of developing coagulation disorders.(2) The fibrinogen test is a crucial tool in assessing clotting ability and monitoring risks associated with bleeding, clotting, and various conditions such as cardiovascular or liver disease.(5)
Lyophilized plasma is processed and dried for use as a reference sample in external quality control programs to ensure the accuracy and stability of test results. Quality control testing is essential in laboratories to guarantee precise results across various testing platforms.(6) Studies have explored lyophilized plasma, especially fibrinogen concentrates, in various medical settings. Clinical trials have compared fibrinogen concentrate to cryoprecipitate in severe trauma patients.(7) Garrick M. et al.(8) conducted a study comparing French lyophilized plasma with fresh frozen plasma and found that French lyophilized plasma achieved higher fibrinogen concentrations. The impact of lyophilization on fibrinogen's coagulation properties is important when calibrating assays with lyophilized plasma. This is crucial in managing major bleeding, where liquid plasma ensures higher plasma levels and accurate coagulation tests.(9) Research on the effects of freeze-drying, freezing, and cryopreservation on plasma components, particularly fibrinogen, has yielded mixed results. Freeze-dried plasma was found to have lower fibrin content compared to fresh plasma, while freezing and freeze-drying were shown to alter the activity of fibrinogen.(10) Despite the limited research supporting the use of fibrinogen concentrate over traditional sources, its potential benefits in managing coagulopathy, particularly in trauma-induced cases, are being recognized.(11)
Lyophilized plasma samples are essential in external quality assurance programs to ensure accurate and consistent fibrinogen assay results for reliable disease diagnosis and treatment.(12) Lyophilized plasma is a valuable tool in supporting lab quality assurance. Quality control products play a crucial role in ensuring precise and accurate results in clinical diagnostics labs.(13)
Laboratory results can be enhanced through rigorous internal control procedures and participation in external quality assessment programs, which help address these challenges. Comparisons between laboratories are crucial for identifying analytical errors not detected by internal controls.(14) So The objective of this research is to investigate the homogeneity and stability of the fibrinogen index in lyophilized plasma, by ISO 13528:2022 and ISO Guide 35:2010 standards.
METHODS
Research method: Experimental research.
Research period: January 2022 to June 2024.
Sampling criteria: Fresh frozen plasma samples, within their expiry date and meeting blood transfusion safety standards as per Circular No. 26/2013/TT-BYT.(15) This circular outlines professional and technical activities related to blood transfusion, including donor selection, blood collection, testing, processing, storage, transportation, management, and use of blood products for treatment; it also covers risk monitoring in blood transfusion, the blood transfusion council at healthcare facilities, and record-keeping and reporting procedures.(15) Fibrinogen analysis results in the sample were less than 4 g/L.
Exclusion criteria: Samples with temperature instability during storage that do not meet the selection criteria.
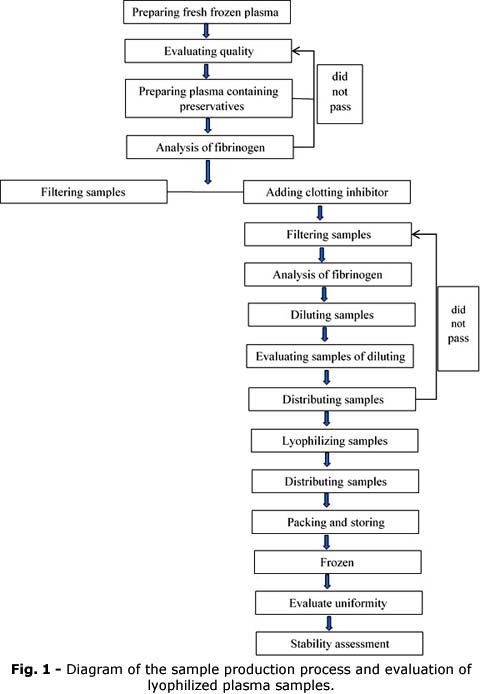
Steps to follow (Fig. 1)
Thaw fresh frozen plasma completely within one hour at room temperature.
Add the preservative mixture and mix thoroughly using a magnetic stirrer in a thermostatically controlled bath for 30 minutes.
Filter the plasma solutions through a 0.22 mm filter paper into a wide-mouth flask. Determine the fibrinogen value in plasma using the Sysmex CS-2000i automatic coagulation system. The system, from Sysmex, Japan, utilizes a multi-wavelength optical measurement method with the following reagents: Actin FSL, LOT: 562674; Calcium Chloride, LOT: 563883; Dade Innovation, LOT: 549783A; Dade Thrombin Reagent, LOT: 565131; Owren's Veronal Buffer, LOT: 569915; CA Clean I, LOT: A1127; CA Clean II, LOT: A1221. Quality control materials include Dade Ci-Trol 1, LOT: 548517 and Dade Ci-Trol 2, LOT: 564845.
Adjust to create a sample set with typical fibrinogen values according to the reagent lot used.
Dispense 1 mL of plasma into a 3 ml brown vial and refrigerate at -80°C for at least 24 hours, then freeze-dry according to the lyophilization procedure at the control center and per the manufacturer's instructions.
Assess homogeneity by randomly selecting ten vials from 200 samples each lot of lyophilized samples at each analysis level, recording, and analyzing using a one-way ANOVA test.
After freezing the batch of samples, evaluate their stability at different stages and temperature milestones. Assess shipping stability over 1, 3, 5, and 7 days and storage stability over 1, 3, and 5 months. Three samples were selected randomly for each stage for evaluation to test the fibrinogen value compared with the initial value using a t-test.
After the lyophilized plasma sets were distributed and packaged, homogeneity assessment was performed by randomly selecting ten vials from each batch, reconstituting them with distilled water, and analyzing each vial twice using the Sysmex CS2000i automatic coagulation system. Subsequently, Excel software was used to conduct one-way ANOVA to evaluate the uniformity of the fibrinogen index in lyophilized plasma.
Once the sample set was confirmed to be homogeneous, its stability was evaluated during transportation and storage using Stata 14.1 software to perform t-tests. These tests assess the stability of the fibrinogen index in lyophilized plasma at a 95% confidence level. A sample was considered stable during storage if the pvalue > 0.05 (p< 0.05 meaning not stable sample). Then the fibrinogen levels at the time of evaluation with the initial homogenization analysis data were compared.
The effects of different storage conditions on raw materials from fresh frozen plasma, stored at -20°C or lower were scanning up to 6 months to ensure sample properties closely resembled plasma. Fresh frozen plasma was selected as the sample source to minimize interference and meet external research requirements.
According to ISO 13528:2022,(16) the proficiency testing provider must ensure that the proficiency testing lots are sufficiently homogeneous and stable to meet the objectives of the proficiency testing program. The study demonstrated that the sample lots achieved uniformity consistent with the one-way ANOVA test method, p> 0.05.
For the standards of external control samples: It is mandatory that samples in the same batch not only be uniform but also maintain the stability of external control parameters over time and across storage temperatures.
Data analysis
Using excel software to conduct one-way ANOVA for evaluating the uniformity of the fibrinogen index in lyophilized plasma and Stata 14.1 to perform t-tests to assess the stability of the fibrinogen index in lyophilized plasma.(17) The t-test was performed on the variable is stability.
Ethical considerations
This study adhered to the ethical standards established by national research committees and organizations, following the principles outlined in the 2024 Declaration of Helsinki and its subsequent amendments or comparable ethical standards. The Biomedical Research Ethics approved the study (approval number 150/HDDD, dated January 17, 2022). Consent was obtained from all blood donors prior to their participation in the study. All donor information was kept confidential, their health was safeguarded, and their mental well-being was not adversely affected.
RESULTS
The results of the homogeneity assessment of plasma after freeze-drying are presented in table 1. This table shows the mean values from two runs (two independently repeated analytical methods) for samples 1, 2, and 3. The p-value, calculated using a one-way ANOVA test, along with the mean and standard deviation (SD), is also provided. According to table 1, the homogeneity of the samples was assessed using a one-way ANOVA test at a significance level of a= 0.05. The fibrinogen values of the standard samples demonstrated homogeneity with p> 0.05 -specifically, p= 0.890- indicating that the required level of uniformity was achieved.
The stability assessment of lyophilized plasma samples containing fibrinogen demonstrated consistent results under both refrigerated and frozen storage, as well as during simulated transport conditions.
Conclusion: Lyophilized samples exhibit robust short-term transport stability and long-term storage stability under both refrigeration and freezing conditions, making them suitable for distribution and usage in external quality control schemes involving fibrinogen assays.
DISCUSSION
Introduced in 1991, Methylene Blue (MB) was the first method developed to inactivate pathogens in labile blood components. MB combined with light (MBL) is capable of inactivating most viruses.(18) However, there is substantial evidence that coagulation factors in plasma, particularly factor VIII, are adversely affected by MB during processing. Consequently, MB was not selected as a preservative or disinfectant.(19) Other reports have indicated that Amotosalen can effectively inactivate pathogens in plasma and maintain coagulation factors, with an average activity level of 81% to 97%.(6,20) Nevertheless, the complexity of the Amotosalen treatment process and the specialized equipment required make it unsuitable for authors Center's conditions.
When investigating the quality of freeze-dried plasma samples, Mok G et al.(21) observed that the freeze-drying process mainly affected FVIII and vWF/RiCoF, which decreased by more than 10% compared to pre-freeze-drying levels, while fibrinogen and other coagulation factors remained relatively stable.(21) The findings are consistent with those of Mok G et al.;(21) in this research on freeze-dried samples, fibrinogen levels remained virtually unchanged, with a percentage difference of only 0.5%.
The freeze-drying process itself can affect plasma samples. Additionally, a common challenge in processing freeze-dried samples is the skill required for reconstitution, which demands meticulous and highly accurate techniques.(22)
When studying the quality of freeze-dried plasma samples, Bux J et al.(23) found that the freeze-drying process only affected FVIII and vWF/RiCoF (decreased by more than 10% compared to before freeze-drying), while fibrinogen and some other coagulation factors remained relatively stable. This study assessed the homogeneity of freeze-dried plasma samples using a single-factor ANOVA test at a= 0.05. The fibrinogen values demonstrated sufficient homogeneity, with a pvalue > 0.05, specifically p= 0.890.
Evaluation of storage stability of sample sets
In the study by Woodhams B. et al.,(24) different storage temperatures were shown to affect the stability duration of samples. At -24°C, the stability of the coagulation factor fibrinogen can extend up to 12 months, and up to 24 months at -70°C.It is evident that fibrinogen's stability duration increases with lower storage temperatures. Therefore, storing ordinary plasma preparations after dilution at -80°C to ensure stability before lyophilization.
Additionally, in another study, a paper-based lateral flow device was utilized to measure fibrinogen levels in lyophilized plasma, further underscoring the potential applications of lyophilized plasma in research and clinical settings.(25) Commercial fibrinogen concentrates, derived from pooled human plasma through cryoprecipitation, are routinely used in clinical settings.(26) These concentrates, which contain lyophilized plasma, can be reconstituted for various uses. Due to Merivaara A et al.,(27) discusses the use of lyophilized plasma in significant trauma scenarios, highlighting its capability to maintain fibrinogen levels and potentially supplement therapeutic plasma or cryoprecipitate. Lyophilization, also known as freeze-drying, is a widely studied method for preserving biological materials and cells.(28) Lyophilized plasma, especially as fibrinogen concentrates, shows potential for applications in trauma treatment, medical research, and biological material preservation. Further research could lead to significant advancements in healthcare and biotechnology.
Saidykhan J et al.(25) studied the stability of lyophilized plasma samples over 12 months at temperatures of 4°C, 25°C, and 40°C, evaluated at 6 and 12 months. The results revealed that fibrinogen concentrations in samples stored at 4°C and 25°C significantly decreased after six months, with p values of 0.04 and 0.02, respectively. The stability was notably lower at 40°C, with p< 0.001. This is consistent with the study of Bux J et al.,(23) which indicated that coagulation factors in lyophilized samples were maintained at normal levels after 24 months of storage at 4°C, suggesting that this temperature is optimal for sample preservation. Similarly, in studies examining the stability of lyophilized plasma at 2-8°C and -20°C, all samples achieved stability over ten weeks at all evaluation points, with p values > 0.05. Saidykhan J et al.(25) suggested that fibrinogen concentration in lyophilized plasma stored at 4°C could be maintained stable for less than six months and was less stable at higher temperatures of 25°C and 40°C.
In this research, samples were assessed for stability at 2-8°C and -20°C over one, three, and five months. Stability was confirmed using t-tests, comparing initial fibrinogen values with those at later intervals. Results showed no significant difference (p> 0.05), indicating the samples remained stable for up to 5 months at both temperatures.
Assessment of the transport stability of the sample set
External quality assessment is a crucial component of quality assurance for laboratory hemostasis assays. Lyophilization of plasma provides stability for labile coagulation factors, facilitating the comparison of results across participating centers. However, the stability of lyophilized samples during transport can be affected by high ambient temperatures, particularly in certain geographic regions.(23)
Jennings I et al.(29) examined the stability of lyophilized plasma samples exposed to an average temperature of 31.9°C, reaching a maximum of 39.7°C over a shipping period of 1 to 8 weeks. The findings revealed that over six weeks, the average change in fibrinogen levels was less than 0.5% at 22°C, less than 2.5% at 30°C, and up to 9% at 37°C. When assessed using a one-way ANOVA, the average fibrinogen values at two, four, and six weeks at 22°C, 30°C, and 37°C showed no statistically significant differences, with ap> 0.05. These results align with this study, indicating that freeze-dried samples can remain stable within one week when temperatures range from 2°C to 30°C during transportation.
In the study by Levy JH et al.(30) the temperature during freeze-drying is critical for preserving fibrinogen. Plasma is frozen at -30°C to -50°C to protect fibrinogen from degradation. However, excessively low or uneven temperatures can alter its structure. During the drying stage, temperatures between -20°C and 0°C under vacuum are used to remove water without damaging fibrinogen. Higher drying temperatures can destabilize it. The freeze-drying process takes 12-24 hours for freezing and 24-48 hours for drying. Insufficient or excessive durations can lead to suboptimal dryness or fibrinogen degradation.
This study demonstrates that lyophilized plasma containing the fibrinogen index is both homogeneous and stable when stored at 2-8°C and -20°C, as well as during transportation based on ISO 13528:2022 and ISO Guide 35:2010 standards. These significant findings pave the way for further research and potential advancements in coagulation testing.
Acknowledgements
We sincerely thank the experts and all participants for volunteering for this study. Thank you to the University of Medicine and Pharmacy at Ho Chi Minh City and Can Tho University of Medicine and Pharmacy in Vietnam for supporting our research.
BIBLIOGRAPHIC REFERENCES
1. Killeen RB, Kaur A, Afzal M. Acute Anemia [Internet]. [Updated 26/02/2025]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537232/
2. Yang J, Li Q, Feng Y, Zeng Y. Iron Deficiency and Iron Deficiency Anemia: Potential Risk Factors in Bone Loss [Internet]. Int J Mol Sci. 2023;24(8):6891. DOI: 10.3390/ijms24086891
3. Gouda AS, Adbelruhman FG, Elbendary RN, Alharbi FA, Alhamrani SQ, Mégarbane BA. Comprehensive insight into the role of zinc deficiency in the renin-angiotensin and kinin-kallikrein system dysfunctions in COVID-19 patients [Internet]. Saudi J Biol Sci. 2021;28 (6):3540-7. DOI: 10.1016/j.sjbs.2021.03.027
4. La QP, Le SH, Nguyen PM, Tran LC. Zinc Deficiency and the Severity of Pneumonia in Vietnamese Children: A Hospital-Based Study [Internet]. Cureus. 2024; 16(7): :e65771. DOI: 10.7759/cureus.65771
5. Testing.com. Fibrinogen Test: test quick guide [Internet]. Onecare Media, Testing.com; 2022. [access: 14/05/2025]. Available at https://www.testing.com/tests/fibrinogen/
6. CAP. New 2025 Surveys and Anatomic Pathology Education Programs [Internet]. [access: 14/05/2025]. Available at: https://www.cap.org/laboratory-improvement/proficiency-testing/new-surveys-and-anatomic-pathology-education-programs
7. Peng HT. Hemostatic agents for prehospital hemorrhage control: a narrative review [Internet]. Mil Med Res. 2020; 25;7:13. DOI: 10.1186/s40779-020-00241-z
8. Mok G, Hoang R, Khan MW, et al. Freeze-dried plasma for major trauma - Systematic review and meta-analysis. J Trauma Acute Care Surg. 2021;90(3):589-602. DOI:10.1097/TA.0000000000003012
9. Rossaint R, Afshari A, Bouillon B, Cerny V, Cimpoesu D, Curry N, et al. The European guideline on management of major bleeding and coagulopathy following trauma: sixth edition [Internet]. Crit Care. 2023; 27(1):80. DOI: 10.1186/s13054-023-04327-7
10. Fernandez-Moure J, Maisha N, Lavik EB, Cannon JW. The chemistry of lyophilized blood products [Internet]. Bioconjug Chem. 2018;29(7):2150--60. DOI: 10.1021/acs.bioconjchem.8b00271
11. Moore EE, Moore HB, Kornblith LZ, Neal MD, Hoffman M, Mutch NJ, et al. Trauma-induced coagulopathy. Nat Rev Dis Primers. 2021;7(1):30. DOI: 10.1038/s41572-021-00264-3
12. Peng HT, Singh K, Rhind SG, da Luz L, Beckett A. Dried Plasma for Major Trauma: Past, Present, and Future. Life 2024, 14, 619. DOI: 10.3390/life14050619
13. Bio-Rad Laboratories. Quality Controls [Internet]. Bio-Rad Laboratories; 2023 [access: 14/05/2025]. Available from: https://www.bio-rad.com/en-vn/category/quality-controls?ID=b11f022f-7ced-4bf0-aaaa-dcdc8affc787
14. Cherie N, Deress T, Berta DM, Chane E, Teketelew BB, Adane K, et al. Navigating Quality Assessment Hurdles in Clinical Laboratory Services: A Comprehensive Review in Resource-Limited Settings [Internet]. Risk Manag Health Policy. 2024;17:497-504. DOI: 10.2147/RMHP.S453020
15. Ministry of Health. Circular guidelines for blood transfusion activities, No. 26/2013/TT-BYT. Vietnam, 2013. Available at: https://thuvienphapluat.vn/van-ban/The-thao-Y-te/Thong-tu-26-2013-TT-BYT-nam-2013-huong-dan-hoat-dong-truyen-mau-214176.aspx
16. International Organization for Standardization. ISO 13528: Statistical methods for proficiency testing by interlaboratory comparison [Internet]. Geneva; 2022. [access: 23/08/2024]. Available at: https://www.iso.org/standard/78879.html
17. StataCorp LLC. Stata and Stata Press are registered trademarks with the World Intellectual Property Organization of the United Nations. StataCorp LLC [Internet]. 2022. [access: 22/08/2024]. Available at: https://www.stata.com/manuals/roneway.pdf
18. Lozano M, Cid J, Müller TH. Plasma treated with methylene blue and light: clinical efficacy and safety profile [Internet]. Transfus Med Rev. 2013;27(4):235-40. DOI: 10.1016/j.tmrv.2013.08.001
19. Cardigan R, Philpot K, Cookson P, Luddington R. Thrombin generation and clot formation in methylene blue-treated plasma and cryoprecipitate [Internet]. Transfusion. 2009;49(4):696-703. DOI: 10.1111/j.1537-2995.2008.02039.x
20. Alarcon Pd, Benjamin R, Dugdale M, Kessler C, Shopnick R, Smith P, et al. Fresh frozen plasma prepared with amotosalen HCl (S-59) photochemical pathogen inactivation: transfusion of patients with congenital coagulation factor deficiencies [Internet]. Transfusion. 2005;45(8):1362-72. DOI: 10.1111/j.1537-2995.2005.00216.x
21. Mok G, Hoang R, Khan MW, Pannell D, Peng H, Tien H, at al. Freeze-dried plasma for major trauma - Systematic review and meta-analysis [Internet]. J Trauma Acute Care Surg. 2021;90(3):589-602. DOI: 10.1097/TA.0000000000003012
22. Nowak D, Jakubczyk E. The Freeze-Drying of Foods-The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials [Internet]. Foods. 2020;9(10):1488. DOI: 10.3390/foods9101488
23. Bux J, Dickhörner D, Scheel E. Quality of freeze-dried (lyophilized) quarantined single-donor plasma [Internet]. Transfusion. 2013;53(12):3203-9. DOI: 10.1111/trf.12191
24. Woodhams B, Girardot O, Blanco MJ, Colesse G, Gourmelinl Y. Stability of coagulation proteins in frozen plasma [Internet]. Blood Coagul Fibrinolysis. 2001;12(4):229-36. DOI: 10.1097/00001721-200106000-00002
25. Saidykhan J, Selevic L, Cinti S, May JE, Killard AJ. Paper-Based Lateral Flow Device for the Sustainable Measurement of Human Plasma Fibrinogen in Low-Resource Settings [Internet]. Anal Chem. 2021;93(41):14007-14013. DOI: 10.1021/acs.analchem.1c03665
26. Kaur J, Jain A. Fibrinogen [Internet]. Treasure Island: StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537184/
27. Merivaara, A., Zini, J., Koivunotko, E., Valkonen, S., Korhonen, O., Fernandes, F. M., et al. Preservation of biomaterials and cells by freeze-drying: Change of paradigm [Internet]. J Control Release. 2021;336:480-498. DOI: 10.1016/j.jconrel.2021.06.042
28. Khaydukova IV, Ivannikova VM, Zhidkov DA, Belikov NV, Peshkova MA, Timashev PS, et al. (2024). Current State and Challenges of Tissue and Organ Cryopreservation in Biobanking. International journal of molecular sciences , 25(20), 11124. https://doi.org/10.3390/ijms252011124
29. Jennings I, Kitchen DP, Woods TAL, Kitchen S, Preston FE, Walker ID. Stability of coagulation proteins in lyophilized plasma [Internet]. Int J Lab Hematol. 2015;37(4):495-502. DOI: 10.1111/ijlh.12318
30. Levy JH, Goodnough LT. How I use fibrinogen replacement therapy in acquired bleeding [Internet]. Blood. 2015;125(9):1387-93. DOI: 10.1182/blood-2014-08-552000
Conflicts of interests
The authors declare that they have no competing interests.
Financial information
The authors declare that no grants were involved in this work.
Authors' contribution
Conceptualization: Thanh Tung Tran, Quang Huy Vu, Thi Hong Nguyen, Phuc
Thi Diem Huynh.
Data
curation: Thi Hong Nguyen.
Formal
analysis: Thanh Tung Tran.
Research:
Thi Hong Nguyen, Phuc Thi Diem Huynh.
Methodology:
Thanh Tung Tran, Quang Huy Vu.
Project
administration: Thi Hong Nguyen.
Supervision:
Quang Huy Vu.
Writing
- Original draft preparation: Phuc Thi Diem Huynh, Thi Hong Nguyen.
Writing:
Review and editing: Thi Hong Nguyen, Thanh Tung Tran, Quang Huy Vu, Phuc
Thi Diem Huynh.
Data Availability
This research data is confidential according to the applicable confidentiality agreements and regulations and, therefore, cannot be publicly displayed or shared. The data are securely stored at Department of Nursing and Medical Technology, Can Tho University of Medicine and Pharmacy. Access to these data requires proper authorization. If you have any questions or need further information, please contact Vu Quang Huy at drvuquanghuy@gmail.com