Systematic Review
Sealing Ability of Resilon as a Root Canal Filling Material: A Systematic Review of In Vitro Studies
Capacidad de sellado del Resilon como material de relleno del conducto radicular: una revisión sistemática de estudios in vitro
Alain Manuel Chaple Gil1* https://orcid.org/0000-0002-8571-4429
Lazareth Liz Ortiz Santiago2 https://orcid.org/0009-0002-3118-0422
Laura Pereda Vázquez2 https://orcid.org/0000-0002-8250-6931
Meylin Santiesteban Velázquez3 https://orcid.org/0009-0008-5935-9313
1Universidad Autónoma de Chile. Facultad de Ciencias de la Salud. Santiago de Chile, Chile.
2Private Practice. United States of America.
3Universidad de Ciencias Médicas de La Habana. Instituto de Ciencias Básicas y Preclínicas Victoria de Girón. La Habana, Cuba.
*Corresponding author. Email: alain.chaple@uautonoma.cl
ABSTRACT
Objective: This systematic review aimed to evaluate the coronal and apical sealing ability of Resilon, a thermoplastic synthetic root canal filling material, compared to gutta-percha under in vitro conditions.
Methods: Following PRISMA-2020 guidelines, a comprehensive search was conducted across four databases (PubMed, Scopus, Web of Science, and Cochrane Library) without restrictions on language or publication date. Although both clinical and in vitro studies were deemed eligible, no clinical studies meeting the inclusion criteria were identified during the literature search. As a result, the final synthesis included exclusively in vitro investigations. Twenty-seven in vitro studies were included after duplicate removal, screening, and eligibility assessment. Two reviewers performed data extraction and risk of bias appraisal independently.
Results: Findings showed that Resilon exhibited superior sealing ability in 17 studies, particularly in terms of reduced microleakage and better adaptation to canal walls, especially when used with methacrylate-based sealers. However, seven studies reported no significant differences between Resilon and gutta-percha, and three studies showed inferior sealing with Resilon, especially in long-term storage or under adverse conditions. Risk of bias analysis revealed methodological concerns in several studies, particularly regarding confounding and outcome measurement. The results suggest that while Resilon may offers short-term advantages in controlled settings, its sealing capacity may decline over time, raising questions about its long-term reliability.
Conclusion: Overall, the evidence supports the potential use of Resilon in specific scenarios where immediate sealing is critical, but further standardized research and clinical trials are necessary to validate its long-term effectiveness and biocompatibility.
Keywords: Root Canal Filling Materials; In Vitro Techniques; Endodontics; Endodontic Obturation.
RESUMEN
Objetivo: Evaluar, mediante revisión sistemática, la capacidad de sellado coronal y apical de Resilon, material sintético termoplástico para obturación radicular, en comparación con la gutapercha en condiciones in vitro.
Métodos: Siguiendo PRISMA-2020, se buscó en PubMed, Scopus, Web of Science y Cochrane Library, sin restricciones de idioma o fecha. Aunque se consideraron estudios clínicos e in vitro, no se hallaron clínicos elegibles, por lo que el análisis incluyó exclusivamente 27 estudios in vitro. Dos revisores realizaron de forma independiente la extracción de datos y la evaluación del riesgo de sesgo.
Resultados: Diecisiete estudios reportaron mejor sellado con Resilon, destacan menor microfiltración y mejor adaptación a las paredes del conducto, especialmente con selladores metacrílicos. Siete estudios no hallaron diferencias significativas y tres evidenciaron peor sellado con Resilon, sobre todo tras almacenamiento prolongado o en condiciones adversas. El análisis de riesgo de sesgo identificó limitaciones metodológicas, particularmente en el control de factores de confusión y medición de resultados.
Conclusiones: Resilon puede ofrecer ventajas inmediatas en entornos controlados, pero su desempeño podría disminuir con el tiempo, lo que genera dudas sobre su confiabilidad a largo plazo. Se requieren investigaciones estandarizadas y ensayos clínicos para confirmar su efectividad y biocompatibilidad.
Palabras clave: Materiales de obturación del conducto radicular; Técnicas in vitro; Endodoncia; Obturación endodóntica.
Received: 21/06/2025
Approved: 12/08/2025
INTRODUCTION
Root canal obturation plays a fundamental role in the long-term success of endodontic treatment by preventing bacterial infiltration and ensuring a tight seal of the root canal system.(1) For decades, gutta-percha, in combination with various sealers, has been the standard filling material, primarily due to its biocompatibility and ease of handling.(2,3)
Resilon emerged in the early 2000s as a thermoplastic synthetic polymer-based obturation material designed to bond with methacrylate-based sealers, forming what was termed a "monoblock" within the canal. The proposed benefits included enhanced coronal and apical sealing, improved resistance to leakage, and potential biodegradability. Despite these theoretical advantages, the actual clinical effectiveness and sealing performance of Resilon have remained controversial. While some in vitro studies suggested superior sealing ability compared to gutta-percha,(4,5) others reported contradictory results, and clinical data on long-term outcomes were limited or inconsistent.(6,7)
Resilon does not have a unique chemical name like a pure compound, as it is a synthetic composite material used for root canal obturation. Its primary composition is based on polycaprolactone, an aliphatic biodegradable polyester that serves as the main matrix component. In addition, it includes methacrylate-based resins such as Bis-GMA and UDMA, commonly found in compatible sealers like Epiphany, as well as radiopaque or bioactive fillers such as barium oxide or titanium dioxide. Therefore, if referring to the chemical name of its principal component, it may be identified as poly(ε-caprolactone); however, as a commercial product, it is generally referred to simply as Resilon, without a specific chemical designation, since it is a complex and patented formulation.(8,9)
Resilon is an endodontic filling material that has gained attention in endodontics due to its potential advantages over traditional gutta-percha. Research has indicated that Resilon may enhance vertical root fracture resistance in endodontically treated teeth when compared to gutta-percha, suggesting it could provide improved structural support.(10,11,12)
In terms of vertical root fracture resistance, Resilon has been shown to increase root strength significantly compared to gutta-percha. A recent meta-analysis reported that Resilon provided better fracture resistance than gutta-percha in combination with AH Plus sealer, with a standardized mean difference (SMD) of 0.77.(11,13)
Despite these promising benefits, some studies have cautioned against overgeneralizing these findings to clinical settings and have emphasized the need for further research to validate these results.(10)
Furthermore, the withdrawal of Resilon from the market raised questions regarding its performance, cost-effectiveness, and reliability, leading to a need for critical reassessment of the existing body of evidence.(8) In light of this context, a systematic and comparative analysis of in vitro studies evaluating the sealing properties of Resilon became imperative to clarify its true advantages and limitations as a root canal filling material.(14)
Research Question: Does the Resilon obturation system provide superior coronal and apical sealing ability compared to other root canal filling materials in clinical and in vitro studies?
The objective of this research was to assess the coronal and apical sealing ability of the Resilon root canal obturation system compared to other obturation materials, by analyzing outcomes from in vitro studies that evaluate microleakage and sealing performance.
METHODS
Design
This systematic review was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 guidelines.(15) The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under ID CRD420251072964.
Eligibility Criteria
This systematic review aimed to evaluate the coronal and apical sealing ability of the Resilon obturation system (Intervention) in comparison with other root canal filling materials such as gutta-percha or bioceramic-based sealers (Comparison). The population included in vitro studies using extracted human or animal teeth prepared for endodontic procedures (Population). The primary outcomes were the extent of coronal or apical microleakage and the overall sealing performance, assessed through various methods including dye penetration, bacterial leakage, and fluid filtration; in clinical studies, treatment success or failure related to sealing quality was also considered (Outcome). This review synthesized the current evidence to determine whether Resilon offers superior, equivalent, or inferior sealing properties compared to conventional materials. Studies were included according to the following criteria:
Types of studies: In vitro studies evaluating microleakage, dye penetration, fluid filtration, bacterial leakage, or related methods; and clinical studies assessing coronal or apical sealing ability or treatment success/failure attributable to coronal/apical leakage of Resilon.
Participants or specimens: Extracted human or animal teeth (in vitro studies), or patients undergoing endodontic treatment (clinical studies).
Intervention: Root canal obturation using the Resilon system.
Comparators: Other root canal filling materials (e.g., gutta-percha) or no comparator.
Outcomes: Quantitative or qualitative assessment of apical and/or coronal microleakage, sealing capacity, or failure due to leakage.
No restrictions were applied regarding the publication date or language of the studies, in order to ensure a comprehensive and unbiased retrieval of all relevant literature on the topic.
Exclusion Criteria
Literature reviews, letters, editorials, commentaries, case reports, abstracts without full text, and studies not evaluating sealing ability (coronal or apical) were excluded.
Information Sources
The following databases were searched: PubMed, Scopus, Web of Science, and Cochrane Library. Additional sources included manual searches in reference lists of included articles.
Search Strategy
A comprehensive search strategy was developed using a combination of MeSH terms and keywords related to “Resilon” (June 4th, 2025; last search performance). To ensure comprehensive retrieval of relevant studies, the search strategy was restricted to the single term "Resilon" applied to the fields of “title”, “abstract”, and “keywords”. This decision was based on the specificity of the topic and the uniqueness of the term "Resilon" as a proprietary brand name of a root canal obturation material. Unlike general terms such as “polycaprolactone-based materials” or “synthetic obturation systems,” which may capture a broad range of unrelated interventions, the term “Resilon” consistently refers to the specific thermoplastic synthetic polymer used in endodontic obturation.
Full search strategy example for Scopus database was TITLE-ABS-KEY (resilon) AND (LIMIT-TO (DOCTYPE , "ar")).
Study Selection
Two reviewers (LPV & MSV) independently screened titles and abstracts. Full texts of potentially relevant articles were assessed for inclusion. Disagreements were resolved by consensus or by consultation with a third reviewer (ACG).
Prior to initiating the study selection process, two independent reviewers underwent a calibration exercise to ensure consistency and reproducibility in the application of eligibility criteria (LPV & MSV). A random sample of 10% of the retrieved records was selected and independently screened by both reviewers. Discrepancies in the inclusion or exclusion decisions were discussed in detail and resolved through consensus. This calibration phase served to refine the operational definitions of the inclusion and exclusion criteria and to minimize inter-reviewer variability.
Following successful calibration, the remaining titles and abstracts were screened independently by the two reviewers (LPV & MSV). Full texts of potentially eligible articles were retrieved and assessed using the same criteria. Any disagreements during this phase were resolved through discussion or, when necessary, by consulting a third reviewer (ACG). The inter-reviewer agreement during the calibration and screening phases was measured using Cohen’s kappa coefficient to quantify the level of concordance.
Data Extraction
A standardized extraction form was used to collect data on authorship, year, country, study design, sample size, methods for leakage/sealing assessment, comparison groups, outcomes, and main findings.
Risk of Bias Assessment
The methodological quality and risk of bias of the included studies were assessed independently by two reviewers using tools appropriate to the study design.
For non-randomized clinical studies, the Risk Of Bias In Non-randomized Studies - of Interventions (ROBINS-I) tool was used, covering bias due to confounding, participant selection, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported result. This adaptation evaluated key methodological aspects including sample size calculation, random allocation of specimens, operator blinding, standardization of procedures, outcome measurement techniques, and statistical analysis. Although no universally accepted tool exists for assessing in vitro studies, this structured approach allowed for a consistent and transparent appraisal of internal validity and experimental rigor.
Any disagreements between reviewers were resolved by consensus or through the consultation of a third reviewer. The risk of bias results was summarized narratively and presented in tabular form to inform the interpretation of findings.
Data Synthesis
All extracted data were organized and managed using a standardized spreadsheet in Microsoft Excel®. The spreadsheet included structured fields for study characteristics, methodological details, outcome measures, and risk of bias assessments. Once data entry was completed and verified for accuracy, the dataset was exported to RStudio® version 2024.12.1 Build 563 for further processing and analysis. R was used to conduct descriptive analyses, visualize data distributions, and explore potential trends or patterns across studies. In accordance with open science principles, the complete dataset used for this review is publicly available in the Mendeley Data repository (https://doi.org/10.17632/fbrnvyxwx3.1), ensuring transparency and reproducibility of the findings.
RESULTS
All studies included in the final synthesis were in vitro investigations, reflecting the current state of experimental evidence regarding the sealing ability and leakage resistance of the Resilon obturation system. Moreover, all selected articles were published in English, which facilitated standardized data extraction and methodological comparison across studies.
Study Selection Process
A comprehensive search strategy was conducted across four major databases: PubMed (n = 37), Scopus (n = 362), Web of Science (n = 235), and Cochrane Library (n = 40), yielding 674 records. Prior to screening, 234 duplicate entries were identified and removed, resulting in 440 unique records subjected to title and abstract screening.
During the screening phase, 246 studies were excluded due to failure to meet the predetermined inclusion criteria based on their titles and abstracts. Consequently, 194 articles were selected for full-text retrieval, all of which were successfully obtained.
Full-text assessment of eligibility led to the exclusion of an additional 167 records. Specifically, 165 studies were excluded for not being related to primary research on the topic, and two articles were excluded due to the unavailability of their full texts. Although the eligibility criteria permitted the inclusion of both clinical and in vitro studies, no clinical studies meeting the predefined selection standards were identified during the search process. It is important to clarify that none of the exclusions at the full-text screening stage were due to the clinical nature of a study failing to meet eligibility requirements. Instead, all excluded articles were discarded for reasons unrelated to study design, such as lack of primary data on sealing performance, absence of outcome measures related to coronal or apical microleakage, or methodological ineligibility. Consequently, the final synthesis was based exclusively on in vitro studies.
Ultimately, 27 articles met all eligibility criteria and were included in the final qualitative synthesis. This rigorous selection process ensured that only studies directly relevant to the coronal and apical sealing or leakage resistance of the Resilon system in clinical and in vitro contexts were retained for comprehensive analysis. (Supplementary File - PRISMA Flowchart).
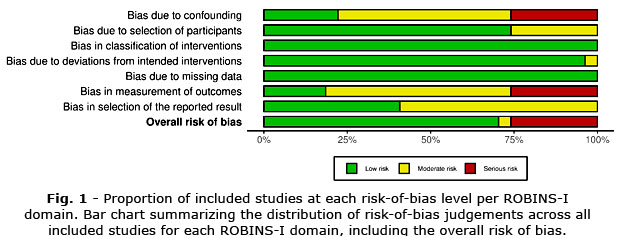
Risk of Bias Assessment
All included studies were evaluated using the ROBINS-I tool to assess the risk of bias across seven domains. As illustrated in figure 1, the majority of studies demonstrated a low risk of bias in the domains of classification of interventions, deviations from intended interventions, and missing data. However, considerable concerns were noted in other areas. Specifically, a serious risk of bias was identified in approximately 30% of studies concerning confounding factors and in 25% for the measurement of outcomes. Additionally, the domain related to the selection of reported results exhibited both moderate and serious risk in a significant proportion of studies, highlighting potential issues in selective outcome reporting.
The detailed domain-level evaluation by individual study is presented in figure 2. According to this analysis, six studies (Onay E et al.,(16) Pitout E et al.,(17) Wang C et al.,(18) Kaya B et al.,(19) Al-Maswary et al.,(20) Eroğlu MG et al.,(21) and Vula V et al.(22)) were classified as having a serious overall risk of bias, primarily due to high concerns in the domains of confounding and outcome measurement. Conversely, several studies, including Paqué F et al.(23) Kececi A et al.,(24) Santos J et al.,(25) and Prithviraj K et al.,(26) achieved a low risk of bias across all evaluated domains, indicating stronger methodological robustness.
These findings underscore variability in methodological quality among the included in vitro studies and emphasize the importance of rigorous design and transparent reporting to reduce potential biases.
Apical and Coronal Seal Performance of Resilon Compared to Gutta-Percha
Among the 27 in vitro studies analyzed, eleven specifically evaluated apical sealing ability. In these, the majority of studies reported that Resilon/Epiphany provided superior apical sealing compared to gutta-percha-based systems. Bodrumlu E et al.,(27) Fathia E et al.,(28) Lambor ET et al.,(29) and Veríssimo DM et al.(30) showed significantly reduced apical dye penetration with Resilon. Studies employing bacterial models such as Prithviraj K et al.(26) and Shipper G et al.(31) also observed lower leakage rates in the Resilon groups. Conversely, long-term storage evaluations by Paqué F et al.(23) revealed that the apical sealing ability of Resilon diminished significantly over time. Similarly, Pasqualini D et al.(32) found more microleakage events in Resilon-filled canals than with gutta-percha.
Five studies assessed coronal sealing, with mixed findings. Al-Maswary AA et al.(20) reported significantly lower coronal dye leakage with Resilon, while Shipper G et al.(31) showed improved resistance to coronal bacterial leakage. However, Santos J et al.(25) demonstrated that gutta-percha combined with AH Plus provided superior coronal sealing under different restoration conditions. De Bruyne M et al.(33) and Zmener O et al.(34) also highlighted that the sealing effectiveness of Resilon was susceptible to degradation or moisture conditions affecting the coronal region (table 1).
Analysis of Resilon and Gutta-Percha Sealing Performance
Due to high methodological heterogeneity among the included in vitro studies particularly in leakage assessment techniques, obturation protocols, storage conditions, and outcome measures a quantitative synthesis or meta-analysis was not feasible. Therefore, the results were synthesized narratively and presented descriptively.
Among the most relevant findings, 17 studies reported that Resilon demonstrated significantly lower leakage or superior sealing performance relative to gutta-percha. For instance, Shipper G et al.(31) found bacterial leakage in only 7–13% of the Resilon samples, compared to 73–93% in gutta-percha groups. Similarly, Fathia E et al.(28) observed a mean apical leakage of 1.06 mm for Resilon, while gutta-percha presented a leakage of 2.53 mm, with a statistically significant difference.
In contrast, seven studies found no statistically significant difference between Resilon and gutta-percha. These results were consistent across various sealing evaluation methods, including dye penetration, fluid filtration, and bacterial leakage models. For example, Almeida J et al.(35) and Kangarlou A et al.(41) found similar sealing performances among all groups tested, including those filled with Resilon or gutta-percha.
Three studies reported higher leakage in the Resilon groups compared to gutta-percha. Paqué F et al.(23) showed that while both materials exhibited similar sealing initially, the Resilon groups demonstrated significantly increased leakage after 16 months of water storage, exceeding 100 µL per 8 hours. Pasqualini D et al.(32) also observed significantly more microleakage events in the Resilon groups using a bacterial detection assay, while Santos J et al.(25) reported that Resilon presented greater leakage than gutta-percha regardless of the coronal restoration employed.
When analyzing methods of leakage assessment, studies using dye penetration techniques frequently showed better sealing with Resilon, as reported by Bodrumlu E et al.(27) In models involving bacterial infiltration, such as those by Shipper G et al.,(31) Resilon groups exhibited reduced bacterial passage compared to gutta-percha, although this was not consistent across all bacterial studies. In time-dependent evaluations, such as those conducted by Paqué F et al.(23) and De Bruyne et al.,(33) Resilon's sealing ability tended to decrease, while gutta-percha retained more consistent performance over time.
These findings contribute directly to the objective of the study by identifying the conditions under which Resilon may offer improved sealing outcomes, as well as those in which its performance aligns with or is inferior to that of gutta-percha. The variation in results depending on the experimental design, evaluation technique, and time frame underscores the importance of standardizing methodologies for more comparable assessments. (Supplementary File - Table).
DISCUSSION
Summary of Main Findings
This systematic review identified and synthesized evidence from 27 in vitro studies comparing the sealing ability of Resilon-based obturation systems with conventional gutta-percha. The majority of studies suggested that Resilon exhibited superior apical and, in some cases, coronal sealing capacity, although this advantage was not consistent across all experiments. A substantial number of investigations found no difference between the two materials, while a smaller proportion indicated inferior performance of Resilon under specific conditions. Risk of bias assessment revealed variability in methodological rigor, with concerns in confounding and outcome measurement domains.
Interpretation and Contribution to Knowledge
The findings reinforce the premise that Resilon’s thermoplastic properties and potential to form a monoblock with methacrylate-based sealers may enhance its initial sealing capacity. These properties could theoretically reduce apical and coronal leakage, thereby improving obturation success. However, discrepancies among studies indicate that such advantages are technique-sensitive and potentially time-limited. The review highlights that while Resilon may achieve better adaptation in controlled laboratory settings, its sealing effectiveness can deteriorate over time or under adverse conditions such as moisture or thermocycling. Thus, the material’s long-term sealing performance remains uncertain.
Comparison with Existing Literature
The findings of the present systematic review align with several earlier evaluations comparing Resilon and gutta-percha regarding their sealing capabilities. Pandey P et al.(4) conducted a systematic review restricted to in vitro studies using the fluid filtration method and reported that although Resilon initially exhibited superior sealing performance, its long-term integrity was compromised, with increased leakage observed over time. In contrast, gutta-percha combined with epoxy-resin-based sealers such as AH Plus demonstrated more consistent sealing behavior throughout extended periods, suggesting better dimensional stability and resistance to degradation under simulated clinical conditions.
Similarly, Shanahan DJ et al.(9) critically reviewed available evidence on Resilon and highlighted the inconsistencies and contradictions across leakage studies. They emphasized that despite some studies suggesting lower microleakage with Resilon, the clinical relevance of such findings remains uncertain due to heterogeneity in experimental models and methodological limitations. They further concluded that Resilon, while promising, could not yet be recommended as a superior or evidence-based replacement for gutta-percha.
Shrestha D et al.(42) echoed these concerns, underscoring that although Resilon/Epiphany systems were originally developed to form a monoblock and theoretically enhance sealing through adhesion to dentin, the actual performance of this system is affected by variables such as polymerization shrinkage, irrigation protocols, and storage conditions. Their review noted that bond strength to dentin remains a concern, and leakage tends to increase with aging of the material, which may limit the longevity of the seal in clinical scenarios.
Conversely, Soares C et al.,(43) in their focused review on retreatment efficacy, addressed the removability of Resilon in comparison with gutta-percha. Their analysis suggested that while Resilon may present advantages in terms of faster and more complete removal using rotary systems and solvents, these benefits do not necessarily imply superior clinical outcomes regarding sealing or treatment success.
Moreover, the in vitro study by Hirai VHG et al.,(44) though not a systematic review, provided comparative insights under controlled laboratory conditions and found that gutta-percha combined with AH Plus consistently produced lower leakage values than Resilon with either AH Plus or Epiphany. This finding reinforces the observations from multiple systematic reviews that have questioned the long-term sealing reliability of Resilon-based systems.
Study Limitations
A primary limitation is the exclusive inclusion of in vitro studies, which restricts extrapolation to clinical outcomes. In vitro conditions cannot fully replicate the complex oral environment, including variables such as occlusal load, bacterial diversity, and patient-specific anatomy. Moreover, heterogeneity in leakage assessment methods (dye, bacterial, fluid, glucose, etc.) and obturation techniques across studies complicates direct comparisons. Variations in tooth type, storage media, and measurement intervals may have introduced further inconsistencies. Risk of bias analysis showed serious concerns in several studies, especially regarding confounding factors and outcome assessment, limiting confidence in some findings.
Implications and Future Directions
The findings of this review suggest that Resilon may serve as a viable alternative to gutta-percha in specific clinical contexts where initial sealing is critical, particularly when used with compatible resin-based sealers and under well-controlled conditions. Nonetheless, concerns regarding its reduced sealing efficacy over time and technique sensitivity necessitate cautious interpretation and application in routine practice. Clinically, its use might be more appropriate in cases where long-term durability is less critical or where optimal coronal protection is guaranteed. From a research standpoint, there is a clear need for standardized in vitro methodologies and rigorously designed randomized clinical trials. Moreover, future investigations should prioritize examining the influence of intraoral factors such as moisture, thermal stress, and retreatment protocols on the long-term performance of Resilon-based obturations.
The absence of eligible clinical studies highlights a critical evidence gap in the current literature, reinforcing the need for future clinical trials to validate the in vitro findings and determine their real-world applicability.
Response to the Research Question
This review sought to compare the sealing ability of Resilon and gutta-percha regarding apical and coronal microleakage. The available evidence suggests that Resilon may exhibit superior sealing in early-stage, in vitro assessments, particularly under ideal experimental conditions. However, this potential advantage does not consistently translate across all studies. In several cases, gutta-percha demonstrated more stable long-term sealing performance, especially under challenging or less controlled conditions. Thus, while Resilon shows potential under specific circumstances, gutta-percha remains the more dependable option overall and the answer to the research question must be viewed in a context-dependent manner.
Resilon appears to offer short-term sealing benefits when paired with appropriate resin-based sealers and used in controlled settings. However, its long-term effectiveness remains uncertain due to inconsistencies in performance related to technique sensitivity and environmental conditions. The current body of evidence highlights considerable variability among studies, which underscores the importance of methodological consistency and clinical validation. To establish the true clinical value of Resilon, future studies must assess its biodegradability, sealing longevity under realistic oral conditions, and comparative effectiveness with modern endodontic sealers and obturation systems.
Future research should focus on evaluating the long-term clinical outcomes of Resilon obturations, assessing biodegradation and sealing effectiveness under dynamic oral conditions, and comparing its performance in conjunction with contemporary sealers and obturation systems. Such efforts are crucial to determining whether Resilon represents a superior alternative to gutta-percha in modern endodontic practice.
BIBLIOGRAPHIC REFERENCES
1. Tait C, Camilleri J, Blundell K. Non-surgical endodontics – obturation [Internet]. British Dental Journal. 2025;238(7):487-96. DOI: https://doi.org/10.1038/s41415-025-8562-1
2. Fernandes AM, Prasad BK, Naik SS, Umesh S. Root canal sealers-a comprehensive review [Internet]. World Journal of Pharmaceutical Research. 2024;13(15):106-16. DOI: https://doi.org/10.20959/wjpr202415-33151
3. Lim M, Jung C, Shin D-H, Cho Y-b, Song M. Calcium silicate-based root canal sealers: a literature review [Internet]. Restor Dent Endod. 2020;45(3):e35. DOI: https://doi.org/10.5395/rde.2020.45.e35
4. Pandey P, Aggarwal H, Tikku AP, Singh A, Bains R, Mishra S. Comparative evaluation of sealing ability of gutta percha and resilon as root canal filling materials- a systematic review [Internet]. Journal of Oral Biology and Craniofacial Research. 2020;10(2):220-6. DOI: https://doi.org/10.1016/j.jobcr.2019.12.004
5. Camilleri J. Sealers and Warm Gutta-percha Obturation Techniques [Internet]. Journal of Endodontics. 2015;41(1):72-8. DOI: https://doi.org/10.1016/j.joen.2014.06.007
6. Strange KA, Tawil PZ, Phillips C, Walia HD, Fouad AF. Long-term Outcomes of Endodontic Treatment Performed with Resilon/Epiphany [Internet]. J Endod. 2019;45(5):507-12. DOI: https://doi.org/10.1016/j.joen.2019.01.019
7. Cotton TP, Schindler WG, Schwartz SA, Watson WR, Hargreaves KM. A Retrospective Study Comparing Clinical Outcomes after Obturation with Resilon/Epiphany or Gutta-Percha/Kerr Sealer [Internet]. Journal of Endodontics. 2008;34(7):789-97. DOI: https://doi.org/10.1016/j.joen.2008.03.018
8. Lotfi M, Ghasemi N, Rahimi S, Vosoughhosseini S, Saghiri MA, Shahidi A. Resilon: a comprehensive literature review [Internet]. J Dent Res Dent Clin Dent Prospects. 2013;7(3):119-30. DOI: https://doi.org/10.5681/joddd.2013.020
9. Shanahan DJ, Duncan HF. Root canal filling using Resilon: A review [Internet]. British Dental Journal. 2011;211(2):81-8. DOI: https://doi.org/10.1038/sj.bdj.2011.573
10. Tan M, Chai Z, Sun C, Hu B, Gao X, Chen Y, et al. Comparative evaluation of the vertical fracture resistance of endodontically treated roots filled with Gutta-percha and Resilon: a meta-analysis of in vitro studies [Internet]. BMC Oral Health. 2018;18(1):107. DOI: https://doi.org/10.1186/s12903-018-0571-x
11. Li G, Li Y, He J, Liu S, Tang J, Jiao T, et al. Comparative assessment of vertical fracture resistance in endodontically treated roots with different obturating systems and techniques: a systematic review and network meta-analysis of in vitro studies [Internet]. BMC Oral Health. 2024;24(1):1439. DOI: https://doi.org/10.1186/s12903-024-05111-x
12. Tanomaru-Filho M, Pinto RVS, Bosso R, Nascimento CA, Berbert FLCV, Guerreiro-Tanomaru JM. Evaluation of the thermoplasticity of gutta-percha and Resilon® using the Obtura II System at different temperature settings [Internet]. International Endodontic Journal. 2011;44(8):764-8. DOI: https://doi.org/10.1111/j.1365-2591.2011.01889.x
13. Attam K, Talwar S. A laboratory comparison of apical leakage between immediate versus delayed post space preparation in root canals filled with Resilon [Internet]. International Endodontic Journal. 2010;43(9):775-81. DOI: https://doi.org/10.1111/j.1365-2591.2010.01742.x
14. Biggs S, Knowles K, Ibarrola J, Pashley D. An in vitro assessment of the sealing ability of resilon/epiphany using fluid filtration [Internet]. J Endod 2006;32(8):759-61. DOI: https://doi.org/10.1016/j.joen.2005.08.013
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews [Internet]. BMJ. 2021;372:n71. DOI: https://doi.org/10.1136/bmj.n71
16. Onay E, Ungor M, Orucoglu H. An in vitro evaluation of the apical sealing ability of a new resin-based root canal obturation system [Internet]. J Endod. 2006;32(10):976-8. DOI: https://doi.org/10.1016/j.joen.2006.05.013
17. Pitout E, Oberholzer TG, Blignaut E, Molepo J. Coronal Leakage of Teeth Root-Filled With Gutta-Percha or Resilon Root Canal Filling Material [Internet]. Journal of Endodontics. 2006;32(9):879-81. DOI: https://doi.org/10.1016/j.joen.2006.02.004
18. Wang C, Debelian G, Teixeira F. Effect of intracanal medicament on the sealing ability of root canals filled with Resilon [Internet]. Journal of endodontics. 2006;32(6):532-6. DOI: https://doi.org/10.1016/j.joen.2005.11.002
19. Kaya B, Kececi A, Belli S. Evaluation of the sealing ability of gutta-percha and thermoplastic synthetic polymer-based systems along the root canals through the glucose penetration model [Internet]. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2007;104(6):e66-73. DOI: https://doi.org/10.1016/j.tripleo.2007.06.024
20. Al-Maswary AA, Alhadainy HA-H, Al-Maweri SA. Coronal microleakage of the resilon and gutta-percha obturation materials with epiphany se sealer: An invitro study [Internet]. Journal of Clinical and Diagnostic Research. 2016;10(5):ZC39-ZC42. DOI: https://doi.org/10.7860/JCDR/2016/17545.7750
21. Eroğlu MG, Bayırlı GŞ. The comparison of dentin adaptation and sealing ability of gutta-percha/AH plus, resilon/epiphany SE, EndoREZ: An in-vitro study [Internet]. Eastern Journal of Medicine. 2017;22(4):155-61. DOI: https://doi.org/10.5505/ejm.2017.86580
22. Vula V, Ajeti N, Kuçi A, Stavileci M, Vula V, Vula V, et al. An In Vitro Comparative Evaluation of Apical Leakage Using Different Root Canal Sealers [Internet]. Medical Science Monitor Basic Research. 2020;26. DOI: https://doi.org/10.12659/MSMBR.928175
23. Paqué F, Sirtes G. Apical sealing ability of Resilon/Epiphany versus gutta-percha/AH Plus: immediate and 16-months leakage [Internet]. International endodontic journal. 2007;40(9):722-9. DOI: https://doi.org/10.1111/j.1365-2591.2007.01298.x
24. Kececi A, Kaya B, Belli S, Kececi AD, Kaya BU, Belli S. Corono-apical Leakage of Various Root Filling Materials Using Two Different Penetration Models-A 3-Month Study [Internet]. Journal Of Biomedical Materials Research Part B-Applied Biomaterials. 2010;92(1):261-7. DOI: https://doi.org/10.1002/jbm.b.31513
25. Santos J, Tjäderhane L, Ferraz C, Zaia A, Alves M, De Goes M, et al. Long-term sealing ability of resin-based root canal fillings [Internet]. International Endodontic Journal. 2010;43(6):455-60. DOI: https://doi.org/10.1111/j.1365-2591.2010.01687.x
26. Prithviraj K, Manjunatha R, Horatti P, Rao N, Gokul S. In Vitro comparison of the microbial leakage of obturation systems: Epiphany with resilon, guttaflow, and ah plus with gutta percha [Internet]. Indian Journal of Dental Research. 2020;31(1):37-41. DOI: https://doi.org/10.4103/ijdr.IJDR_98_18
27. Bodrumlu E, Tunga U. Apical leakage of Resilon™ obturation material [Internet]. Journal of Contemporary Dental Practice. 2006;7(4):045-52. DOI: https://doi.org/10.5005/jcdp-7-4-45
28. Fathia E, Abu-Bakr NH, Yahia I. A comparative study of the microleakage of resilon/epiphany and gutta-percha/AH-Plus obturating systems [Internet]. Iranian Endodontic Journal. 2012 [acceso: 11/04/2025];7(3):139-43. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC3467139/
29. Lambor RT, De Noronha De Ataide I, Chalakkal P, Akkara F, Shariff SA, Fernandes KS. An in vitro comparison between the apical sealing abilities of resilon with Epiphany® sealer and gutta-percha with AH plus sealer [Internet]. Indian Journal of Dental Research. 2012;23(5):694. DOI: https://doi.org/10.4103/0970-9290.107415
30. Veríssimo DM, Sampaio do Vale M, Monteiro AJ. Comparison of Apical Leakage between Canals Filled with Gutta-Percha/AH-Plus and the Resilon/Epiphany System, When Submitted to Two Filling Techniques [Internet]. J Endod. 2007;33(3):291-4. DOI: https://doi.org/10.1016/j.joen.2006.10.014
31. Shipper G, Ørstavik D, Teixeira FB, Trope M. An evaluation of microbial leakage in roots filled with a thermoplastic synthetic polymer-based root canal filling material (Resilon) [Internet]. J Endod. 2004;30(5):342-7. DOI: https://doi.org/10.1097/00004770-200405000-00009
32. Pasqualini D, Scotti N, Mollo L, Berutti E, Angelini E, Migliaretti G, et al. Microbial leakage of gutta-percha and Resilon™ root canal filling material: A comparative study using a new homogeneous assay for sequence detection [Internet]. Journal Of Biomaterials Applications. 2008;22(4):337-52. DOI: https://doi.org/10.1177/0885328207077411
33. De Bruyne M, De Moor R, De Bruyne MAA, De Moor RJG. Long-term sealing ability of Resilon apical root-end fillings [Internet]. International Endodontic Journal. 2009;42(10):884-92. DOI: https://doi.org/10.1111/j.1365-2591.2009.01583.x
34. Zmener O, Pameijer C, Serrano S, Vidueira M, Macchi R, Zmener O, et al. Significance of moist root canal dentin with the use of methacrylate-based endodontic sealers: An in vitro coronal dye leakage study [Internet]. Journal Of Endodontics. 2008;34(1):76-9. DOI: https://doi.org/10.1016/j.joen.2007.10.012
35. Almeida J, Gomes B, Ferraz C, Souza-Filho F, Zaia A. Filling of artificial lateral canals and microleakage and flow of five endodontic sealers [Internet]. International endodontic journal. 2007;40(9):692-9. DOI: https://doi.org/10.1111/j.1365-2591.2007.01268.x
36. Baumgartner G, Zehnder M, Paqué F. Enterococcus faecalis type strain leakage through root canals filled with Gutta-Percha/AH plus or Resilon/Epiphany [Internet]. J Endod 2007;33(1):45-7. DOI: https://doi.org/10.1016/j.joen.2006.08.002
37. Hammad M, Qualtrough A, Silikas N, Hammad M, Qualtrough A, Silikas N. Evaluation of Root Canal Obturation: A Three-dimensional In Vitro Study [Internet]. J Endod. 2009;35(4):541-4. DOI: https://doi.org/10.1016/j.joen.2008.12.021
38. Kqiku L, Städtler P, Gruber H, Baraba A, Anic I, Miletic I, et al. Active versus passive microleakage of Resilon/Epiphany and gutta-percha/AH Plus [Internet]. Australian Endodontic Journal. 2011;37(3):141-6. DOI: https://doi.org/10.1111/j.1747-4477.2010.00238.x
39. Nawal R, Parande M, Sehgal R, Rao N, Naik A, Nawal RR, et al. A comparative evaluation of 3 root canal filling systems [Internet]. Oral Surgery Oral Medicine Oral Pathology Oral Radiology And Endodontology. 2011;111(3):387-93. DOI: https://doi.org/10.1016/j.tripleo.2010.09.070
40. Abdo SB, Darrat AA, Masudi SM, Luddin N, Husien A. Sealing ability of gutta-percha/nano HA versus resilon/epiphany after 20 months using an electrochemical model - an in vitro study [Internet]. Brazilian Journal of Oral Sciences. 2012 [acceso: 11/04/2025];11(3):387-91. Available from: https://periodicos.sbu.unicamp.br/ojs/index.php/bjos/article/view/8641375
41. Kangarlou A, Dianat O, Esfahrood Z, Asharaf H, Zandi B, Eslami G, et al. Bacterial leakage of GuttaFlow-filled root canals compared with Resilon/Epiphany and Gutta-percha/AH26-filled root canals [Internet]. Australian Endodontic Journal. 2012;38(1):10-3. DOI: https://doi.org/10.1111/j.1747-4477.2010.00267.x
42. Shrestha D, Wei X, Wu WC, Ling JQ. Resilon: A methacrylate resin-based obturation system [Internet]. J Dent Sci. 2010;5(2):47-52. DOI: https://doi.org/10.1016/S1991-7902(10)60008-6
43. Soares C, Maia C, Vale F, Gadê-Neto C, Carvalho L, Oliveira H, et al. Comparison of endodontic retreatment in teeth obturated with resilon or gutta-percha: A review of literature [Internet]. Iranian Endodontic Journal. 2015;10(4):221-5. DOI: https://doi.org/10.7508/iej.2015.04.002
44. Hirai VHG, da Silva Neto UX, Westphalen VPD, Perin CP, Carneiro E, Fariniuk LF. Comparative analysis of leakage in root canal fillings performed with gutta-percha and Resilon cones with AH Plus and Epiphany sealers [Internet]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(2):e131-e5. DOI: https://doi.org/10.1016/j.tripleo.2009.09.038
Conflict of interest
The authors declared no conflict of interest.
Funding
This research has not received fundings or economic supports from any institution.
Authorship contribution
Conceptualization: Alain Chaple Gil.
Data curation: Alain Chaple Gil, Meylin Santiesteban Velázquez, Lazareth Liz Ortiz Santiago.
Data Analysis: Alain Chaple Gil, Meylin Santiesteban Velázquez, Laura Pereda Vázquez, Lazareth Liz Ortiz Santiago.
Research: Alain Chaple Gil, Meylin Santiesteban Velázquez, Laura Pereda Vázquez, Lazareth Liz Ortiz Santiago.
Methodology: Alain Chaple Gil, Laura Pereda Vázquez, Lazareth Liz Ortiz Santiago.
Project administration: Alain Chaple Gil.
Software: Alain Chaple Gil, Lazareth Liz Ortiz Santiago.
Supervision: Alain Chaple Gil.
Validation: Alain Chaple Gil, Meylin Santiesteban Velázquez, Laura Pereda Vázquez, Lazareth Liz Ortiz Santiago.
Display: Alain Chaple Gil, Meylin Santiesteban Velázquez, Laura Pereda Vázquez, Lazareth Liz Ortiz Santiago.
Writing - original draft: Alain Chaple Gil, Meylin Santiesteban Velázquez, Laura Pereda Vázquez, Lazareth Liz Ortiz Santiago.
Drafting - revision and editing: Alain Chaple Gil, Meylin Santiesteban Velázquez, Laura Pereda Vázquez, Lazareth Liz Ortiz Santiago.
Data Availability
Mendeley Data repository: https://doi.org/10.17632/fbrnvyxwx3.1
Supplementary files:
PRISMA flowchart; table with Summary of In Vitro Studies Comparing Sealing Ability of Resilon/Epiphany and Gutta-Percha Systems (PDF). Available from: https://revmedmilitar.sld.cu/index.php/mil/libraryFiles/downloadPublic/92
Figure 1 with original resolution. Available from: https://revmedmilitar.sld.cu/index.php/mil/libraryFiles/downloadPublic/93
Figure 2 with original resolution. Available from: https://revmedmilitar.sld.cu/index.php/mil/libraryFiles/downloadPublic/94